Page 151 - Radiochemistry and nuclear chemistry
P. 151
136 Radiochemistry and Nuclear Chemistry
energy of the/3-particle is lost by ionization and half by excitation. The track formed is
further discussed in w
The specific ionization from a B-particle is much lower than that from a heavy ion as can
be seen in Figure 6.7a. This is due to the fact that for the same initial energy, B-particles
have much greater velocity than have or-particles or protons because their mass is very
much smaller than the mass of the heavy particles. This greater velocity results in a
correspondingly lower ionization and gives a much longer range to/~-particles. The erratic
path observed for/~-particles in Figure 6.5c is a result of the large energy transfer and the
resulting large deflections involved in the encounters with the orbital electrons. However,
at very high energies the/~-particles have straight paths as a result of the fact that very
energetic B-particles have a momentum considerably in excess of that of the orbital
electron.
6.4.2. Bremsstrahlung
As a/3-particle approaches an atomic nucleus, it is attracted by the positive field of the
nucleus and deflected from its path. The deflection results in an acceleration that, according
to classical electrodynamics, leads to emission of electromagnetic radiation (Fig. 6.8c).
Therefore the encounter with the positive charge of the nuclear field decreases the energy
of the/3-particle by an amount exactly equal to the amount of electromagnetic radiation
emitted. This radiation is known as bremsstrahlung (braking radiation). The loss of energy
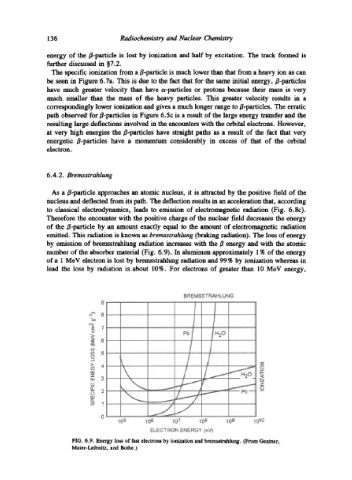
by emission of bremsstrahlung radiation increases with the/~ energy and with the atomic
number of the absorber material (Fig. 6.9). In aluminum approximately 1% of the energy
of a 1 MeV electron is lost by bremsstrahlung radiation and 99 % by ionization whereas in
lead the loss by radiation is about 10%. For electrons of greater than 10 MeV energy,
FIG. 6.9. Energy loss of fast electrons by ionization and bremsstrahlung. (From Gentner,
Maier-Leibnitz, and Bothe.)