Page 153 - Radiochemistry and nuclear chemistry
P. 153
138 Radiochemistry and Nuclear Chemistry
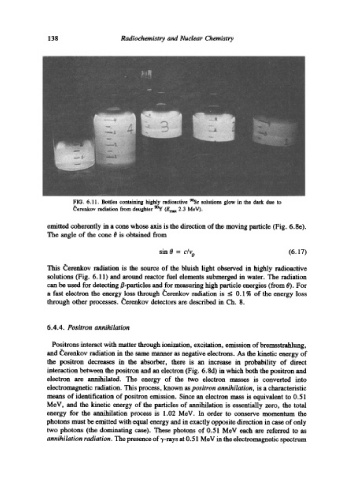
FIG. 6.11. Bottles containing highly radioactive 9~ solutions glow in the dark due to
~erenkov radiation from daughter 90y (Errmx 2.3 MeV).
emitted coherently in a cone whose axis is the direction of the moving particle (Fig. 6.8e).
The angle of the cone 0 is obtained from
sin 0 = C/Vp (6.17)
This ~erenkov radiation is the source of the bluish light observed in highly radioactive
solutions (Fig. 6.11) and around reactor fuel dements submerged in water. The radiation
can be used for detecting #-particles and for measuring high particle energies (from 0). For
a fast electron the energy loss through r radiation is _< 0.1% of the energy loss
through other processes. r detectors are described in Ch. 8.
6.4.4. Positron annihilation
Positrons interact with matter through ionization, excitation, emission of bremsstrahlung,
and ~erenkov radiation in the same manner as negative electrons. As the kinetic energy of
the positron decreases in the absorber, there is an increase in probability of direct
interaction between the positron and an electron (Fig. 6.8d) in which both the positron and
electron are annihilated. The energy of the two electron masses is converted into
electromagnetic radiation. This process, known as positron annihilation, is a characteristic
means of identification of positron emission. Since an electron mass is equivalent to 0.51
MeV, and the kinetic energy of the particles of annihilation is essentially zero, the total
energy for the annihilation process is 1.02 MeV. In order to conserve momentum the
photons must be emitted with equal energy and in exactly opposite direction in case of only
two photons (the dominating case). These photons of 0.51 MeV each are referred to as
annihilation radiation. The presence of'y-rays at 0.51 MeV in the electromagnetic spectrum