Page 152 - Radiochemistry and nuclear chemistry
P. 152
Absorption of Nuclear Radiation 137
II
~ i
uJ
.=1
_o
.o
O O BREI~ST
.i
0 0.060 0.100 0.150 0.200
PARTICLE ENERGY (MeV)
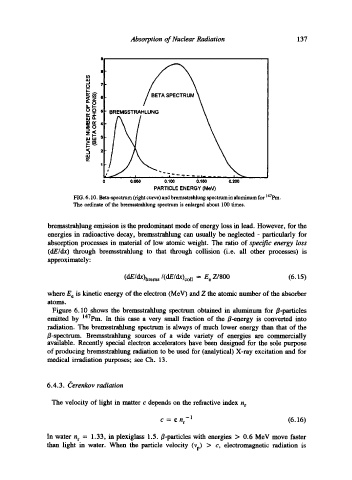
FIG. 6.10. Beta-spectrum (right curve)and bremsstrahlung spectrum in aluminum for 147pm.
The ordinate of the bremsstrahlung spectrum is enlarged about 100 times.
bremsstrahlung emission is the predominant mode of energy loss in lead. However, for the
energies in radioactive decay, bremsstrahlung can usually be neglected - particularly for
absorption processes in material of low atomic weight. The ratio of specific energy loss
(dE/dx) through bremsstrahlung to that through collision (i.e. all other processes) is
approximately:
(dE/dX)brems/(dE/dX)col I ~. E e Z/800 (6.15)
where E e is kinetic energy of the electron (MeV) and Z the atomic number of the absorber
atoms.
Figure 6.10 shows the bremsstrahlung spectrum obtained in aluminum for/~-particles
emitted by 147pm. In this case a very small fraction of the/~-energy is converted into
radiation. The bremsstrahlung spectrum is always of much lower energy than that of the
/~-spectrum. Bremsstrahlung sources of a wide variety of energies are commercially
available. Recently special electron accelerators have been designed for the sole purpose
of producing bremsstrahlung radiation to be used for (analytical) X-ray excitation and for
medical irradiation purposes; see Ch. 13.
6.4.3. C.erenkov radiation
The velocity of light in matter c depends on the refractive index n r
c= cn r 1 (6.16)
In water n r = 1.33, in plexiglass 1.5./~-particles with energies > 0.6 MeV move faster
than light in water. When the particle velocity (vp) > c, electromagnetic radiation is