Page 148 - Radiochemistry and nuclear chemistry
P. 148
Absorption of Nuclear Radiation 133
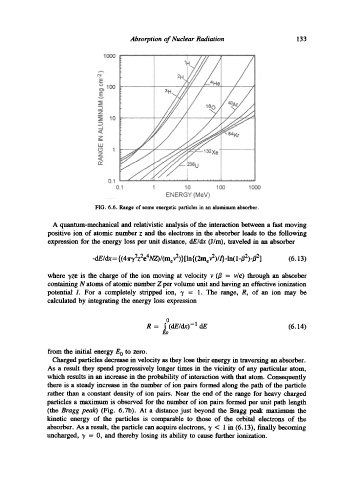
FIG. 6.6. Range of some energetic particles in an aluminum absorber.
A quantum-mechanical and relativistic analysis of the interaction between a fast moving
positive ion of atomic number z and the electrons in the absorber leads to the following
expression for the energy loss per unit distance, dE/dx (J/m), traveled in an absorber
-dE/d.r= {(47r,),2z2e4NZ)/fmev2)} [ln{(2meV2)//}-In(l-/32)-/32] (6.13)
where vze is the charge of the ion moving at velocity v (/~ = v/c) through an absorber
containing N atoms of atomic number Z per volume unit and having an effective ionization
potential I. For a completely stripped ion, 7 = 1. The range, R, of an ion may be
calculated by integrating the energy loss expression
0
R = I (dE/dx)-l dE (6.14)
Eo
from the initial energy E 0 to zero.
Charged particles decrease in velocity as they lose their energy in traversing an absorber.
As a result they spend progressively longer times in the vicinity of any particular atom,
which results in an increase in the probability of interaction with that atom. Consequently
there is a steady increase in the number of ion pairs formed along the path of the particle
rather than a constant density of ion pairs. Near the end of the range for heavy charged
particles a maximum is observed for the number of ion pairs formed per unit path length
(the Bragg peak) (Fig. 6.7b). At a distance just beyond the Bragg peak maximum the
kinetic energy of the particles is comparable to those of the orbital electrons of the
absorber. As a result, the particle can acquire electrons, 7 < 1 in (6.13), finally becoming
uncharged, "y = 0, and thereby losing its ability to cause further ionization.