Page 160 - Radiochemistry and nuclear chemistry
P. 160
Absorption of Nuclear Radiation 145
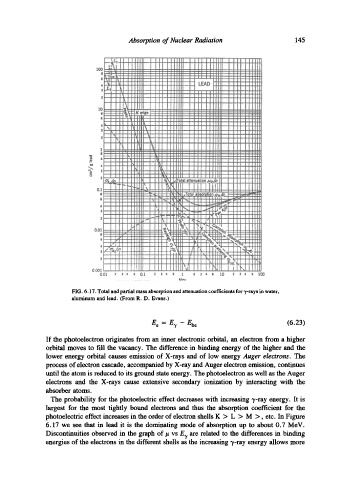
FIG. 6.17. Total and partial mass absorption and attenuation coefficients for v-rays in water,
aluminum and lead. (From R. D. Evans.)
E e = E3, - Ebe (6.23)
If the photoelectron originates from an inner electronic orbital, an electron from a higher
orbital moves to fill the vacancy. The difference in binding energy of the higher and the
lower energy orbital causes emission of X-rays and of low energy Auger electrons. The
process of electron cascade, accompanied by X-ray and Auger electron emission, continues
until the atom is reduced to its ground state energy. The photoelectron as well as the Auger
electrons and the X-rays cause extensive secondary ionization by interacting with the
absorber atoms.
The probability for the photoelectric effect decreases with increasing "y-ray energy. It is
largest for the most tightly bound electrons and thus the absorption coefficient for the
photoelectric effect increases in the order of electron shells K > L > M >, etc. In Figure
6.17 we see that in lead it is the dominating mode of absorption up to about 0.7 MeV.
Discontinuities observed in the graph of/t vs E7 are related to the differences in binding
energies of the electrons in the different shells as the increasing -/-ray energy allows more