Page 169 - Radiochemistry and nuclear chemistry
P. 169
Absorption of Nuclear Radiation 153
CHEM. SHIFT
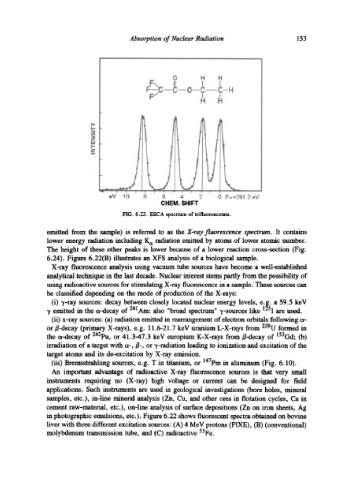
FIG. 6.23. ESCA spectrum of trifluoroacetate.
emitted from the sample) is referred to as the X-ray fluorescence spectrum. It contains
lower energy radiation including K~ radiation emitted by atoms of lower atomic number.
The height of these other peaks is lower because of a lower reaction cross-section (Fig.
6.24). Figure 6.22(B) illustrates an XFS analysis of a biological sample.
X-ray fluorescence analysis using vacuum tube sources have become a well-established
analytical technique in the last decade. Nuclear interest stems partly from the possibility of
using radioactive sources for stimulating X-ray fluorescence in a sample. These sources can
be classified depending on the mode of production of the X-rays:
(i) ~,-ray sources: decay between closely located nuclear energy levels, e.g. a 59.5 keV
-y emitted in the a-decay of 241 Am: also broad spectrum -y-sources like 125 I are used.
(ii) x-ray sources: (a) radiation emitted in rearrangement of electron orbitals following or-
or B-decay (primary X-rays), e.g. 11.6-21.7 keV uranium L-X-rays from 23gU formed in
the c~-decay of 242pu, or 41.3-47.3 keV europium K-X-rays from fl-decay of 153Gd; (b)
irradiation of a target with a-, ~-, or "y-radiation leading to ionization and excitation of the
target atoms and its de-excitation by X-ray emission.
(iii) Bremsstrahlung sources, e.g. T in titanium, or 147pm in aluminum (Fig. 6.10).
An important advantage of radioactive X-ray fluorescence sources is that very small
instruments requiring no (X-ray) high voltage or current can be designed for field
applications. Such instruments are used in geological investigations (bore holes, mineral
samples, etc.), in-line mineral analysis (Zn, Cu, and other ores in flotation cycles, Ca in
cement raw-material, etc.), on-line analysis of surface depositions (Zn on iron sheets, Ag
in photographic emulsions, etc.). Figure 6.22 shows fluorescent spectra obtained on bovine
liver with three different excitation sources: (A) 4 MeV protons (PIXE), (B) (conventional)
molybdenum transmission tube, and (C) radioactive 55Fe.