Page 170 - Radiochemistry and nuclear chemistry
P. 170
154 Radiochemistry and Nuclear Chemistry
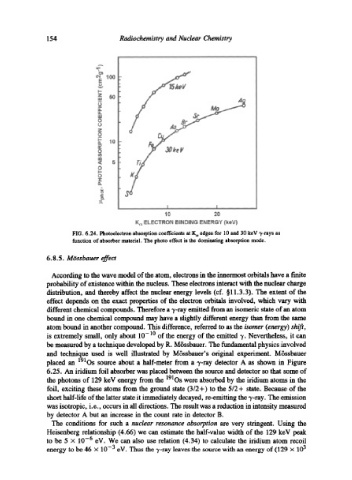
FIG. 6.24. Photoelectron absorption coefficients at K a edges for 10 and 30 keV 3,-rays as
function of absorber material. The photo effect is the dominating absorption mode.
6.8.5. MOssbauer effect
According to the wave model of the atom, electrons in the innermost orbitals have a finite
probability of existence within the nucleus. These electrons interact with the nuclear charge
distribution, and thereby affect the nuclear energy levels (cf. w The extent of the
effect depends on the exact properties of the electron orbitals involved, which vary with
different chemical compounds. Therefore a 7-ray emitted from an isomeric state of an atom
bound in one chemical compound may have a slightly different energy than from the same
atom bound in another compound. This difference, referred to as the isomer (energy) shift,
is extremely small, only about 10-10 of the energy of the emitted 7. Nevertheless, it can
be measured by a technique developed by R. MSssbauer. The fundamental physics involved
and technique used is well illustrated by MSssbauer's original experiment. MSssbauer
placed an 19lOs source about a half-meter from a 7-ray detector A as shown in Figure
6.25. An iridium foil absorber was placed between the source and detector so that some of
the photons of 129 keV energy from the 191Os were absorbed by the iridium atoms in the
foil, exciting these atoms from the ground state (3/2 +) to the 5/2 + state. Because of the
short half-life of the latter state it immediately decayed, re-emitting the 7-ray. The emission
was isotropic, i.e., occurs in all directions. The result was a reduction in intensity measured
by detector A but an increase in the count rate in detector B.
The conditions for such a nuclear resonance absorption are very stringent. Using the
Heisenberg relationship (4.66) we can estimate the half-value width of the 129 keV peak
to be 5 • 10 -6 eV. We can also use relation (4.34) to calculate the iridium atom recoil
energy to be 46 • 10 -3 eV. Thus the -y-ray leaves the source with an energy of (129 x 103