Page 173 - Radiochemistry and nuclear chemistry
P. 173
Absorption of Nuclear Radiation 157
+60
--AmF 3
+5o - k,,.a(c2o,~
14AmEOT&
NPX 3
+40 X- F, CI, Br
"P2S:
+30 . -- PulU 4
A
"7
w
+20-
v -- Puk2
I-- ~4 _ p~
IJ. +10 -
3:
(/) ~a
--a-Pu
W -~.o 2
5: O- a-.p -p.F, 2
0 - I)-Pu N~4..-"
KNpO2r 3 -
-K~)2CO:
-10 -
-20-
-30-
-40
(o) III IV v
OXIDATION STATE
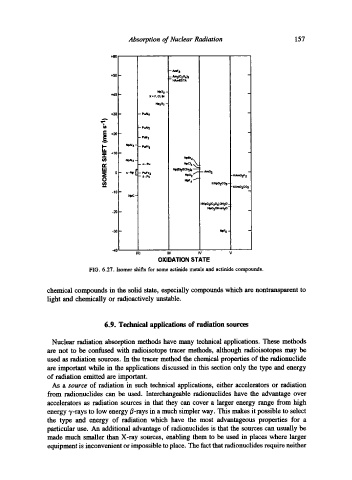
FIG. 6.27. Isomer shiRs for some actinide metals and actinide compounds.
chemical compounds in the solid state, especially compounds which are nontransparent to
light and chemically or radioactively unstable.
6.9. Technical applications of radiation sources
Nuclear radiation absorption methods have many technical applications. These methods
are not to be confused with radioisotope tracer methods, although radioisotopes may be
used as radiation sources. In the tracer method the chemical properties of the radionuclide
are important while in the applications discussed in this section only the type and energy
of radiation emitted are important.
As a source of radiation in such technical applications, either accelerators or radiation
from radionuclides can be used. Interchangeable radionuclides have the advantage over
accelerators as radiation sources in that they can cover a larger energy range from high
energy "y-rays to low energy ~/-rays in a much simpler way. This makes it possible to select
the type and energy of radiation which have the most advantageous properties for a
particular use. An additional advantage of radionuclides is that the sources can usually be
made much smaller than X-ray sources, enabling them to be used in places where larger
equipment is inconvenient or impossible to place. The fact that radionuclides require neither