Page 278 - Radiochemistry and nuclear chemistry
P. 278
262 Radiochemistry and Nuclear Chemistry
(b) Solvent extraction
As described in w the solvent extraction technique requires (i) a liquid two-phase
system consisting of an organic solvent in contact with an aqueous solution, and (ii) the
presence of an extractant (commonly a weak organic acid, abbreviated HA), which reacts
with the metal ion to form an uncharged metal-organic complex MA z, that preferentially
dissolves in the organic phase. The distribution of the metal between the organic and
aqueous phases can be shown to be fit the relation
DM = KDC/~z [A-]Z / y:" 'Sn [A-]n (9.23a)
where D M is the distribution ratio of the metal as defined by eqn. (9.6), and Ktx ~ is the
distribution constant of the uncharged complex MA z. [A-] is referred to as the free ligand
ion concentration; using trace metal concentration, [A'] is easily calculated from the amount
of acid, HA, added, its dissociation constant K a, pH and the liquid volumes. From
measurements of D M as a function of [A-], the formation constants/~n for the complexes
MAn z-n are calculated.
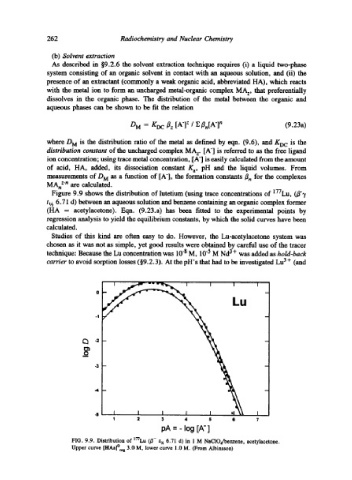
Figure 9.9 shows the distribution of lutetium (using trace concentrations of 177Lu, (/~--y
tt,~ 6.71 d) between an aqueous solution and benzene containing an organic complex former
(HA = acetylacetone). Eqn. (9.23.a) has been fitted to the experimental points by
regression analysis to yield the equilibrium constants, by which the solid curves have been
calculated.
Studies of this kind are often easy to do. However, the Lu-acetylacetone system was
chosen as it was not as simple, yet good results were obtained by careful use of the tracer
technique: Because the Lu concentration was 10 -8 M, 10 -5 M Nd 3 + was added as hoM-back
carrier to avoid sorption losses (w At the pH's that had to be investigated Lu 3+ (and
Lu
O
m
-6
1 2 3 4 5 6 7
pA =- log [A-]
FIG. 9.9. Distribution of t'Lu (B- t,~ 6.71 d) in l M NaCIO4/benzene, acetylacetone.
Upper curve [HAa]~ 3.0 M, lower curve 1.0 M. (From Albinsson)