Page 87 - Radiochemistry and nuclear chemistry
P. 87
76 Radiochemistry and Nuclear Chemistry
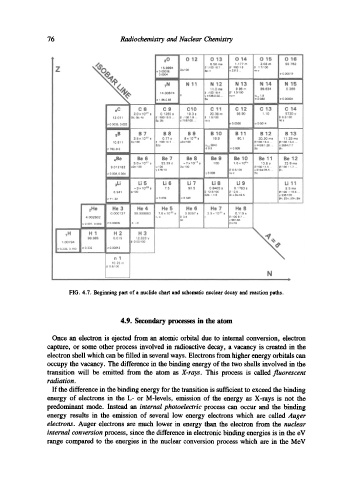
FIG. 4.7. Beginning part of a nuclide chart and schematic nuclear decay and reaction paths.
4.9. Secondary processes in the atom
Once an electron is ejected from an atomic orbital due to internal conversion, electron
capture, or some other process involved in radioactive dec~y, a vacancy is created in the
electron shell which can be filled in several ways. Electrons from higher energy orbitals can
occupy the vacancy. The difference in the binding energy of the two shells involved in the
transition will be emitted from the atom as X-rays. This process is called fluorescent
radiation.
If the difference in the binding energy for the transition is sufficient to exceed the binding
energy of electrons in the L- or M-levels, emission of the energy as X-rays is not the
predominant mode. Instead an internal photoelectric process can occur and the binding
energy results in the emission of several low energy electrons which are called Auger
electrons. Auger electrons are much lower in energy than the electron from the nuclear
internal conversion process, since the difference in electronic binding energies is in the eV
range compared to the energies in the nuclear conversion process which are in the MeV