Page 92 - Radiochemistry and nuclear chemistry
P. 92
Unstable Nuclei and Radioactive Decay 81
radioactive decay, ff commonly has a value between 0.01 and 0.5. Equation (4.45) is only
valid provided At ,r tt h (in which case AN ,~ N), where At is the time of measurement; this
is the normal situation; cf. (4.40b).
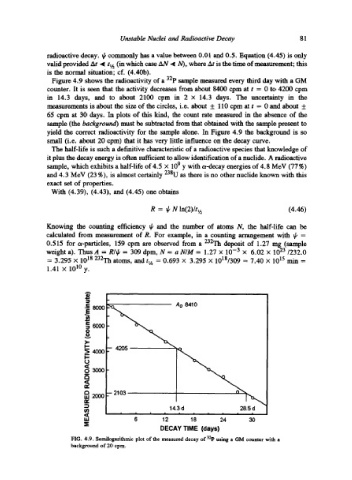
Figure 4.9 shows the radioactivity of a 32p sample measured every third day with a GM
counter. It is seen that the activity decreases from about 8400 cpm at t = 0 to 4200 cpm
in 14.3 days, and to about 2100 cpm in 2 • 14.3 days. The uncertainty in the
measurements is about the size of the circles, i.e. about + 110 cpm at t = 0 and about +
65 cpm at 30 days. In plots of this kind, the count rate measured in the absence of the
sample (the background) must be subtracted from that obtained with the sample present to
yield the correct radioactivity for the sample alone. In Figure 4.9 the background is so
small (i.e. about 20 cpm) that it has very little influence on the decay curve.
The half-life is such a definitive characteristic of a radioactive species that knowledge of
it plus the decay energy is often sufficient to allow identification of a nuclide. A radioactive
sample, which exhibits a half-life of 4.5 • 109 y with a-decay energies of 4.8 MeV (77 %)
and 4.3 MeV (23 %), is almost certainly 238U as there is no other nuclide known with this
exact set of properties.
With (4.39), (4.43), and (4.45) one obtains
R = ~, N ln(2)/t~/= (4.46)
Knowing the counting efficiency ~ and the number of atoms N, the half-life can be
calculated from measurement of R. For example, in a counting arrangement with ~ =
0.515 for a-particles, 159 cpm are observed from a 232Th deposit of 1.27 mg (sample
weight a). Thus A - R/~k -- 309 dpm, N - a N/M - 1.27 x 10 -3 x 6.02 x 1023/232.0
= 3.295 x 1018 232Th atoms, and t~ h = 0.693 x 3.295 x 1018/309 = 7.40 x 1015 min =
1.41 • 101~ y.
.=
A
r
9 8000 Ao 8410
E
c
= 6(xx)
o o
>-
i
P
(J
<C
o3oo0
-- 2103
uj 2(X)0
n,
14.3 d 28.5 d
" " ! I I II I I I I
uJ 6 12 18 24 30
=E
DECAY TIME (days)
FIG. 4.9. Semilogarithmic plot of the measured decay of 32p using a GM counter with a
background of 20 cpm.