Page 95 - Radiochemistry and nuclear chemistry
P. 95
84 Radiochemistry and Nuclear Chemistry
4.14. Branching decay
Several times in this chapter the possibility of competing modes of decay has been noted;
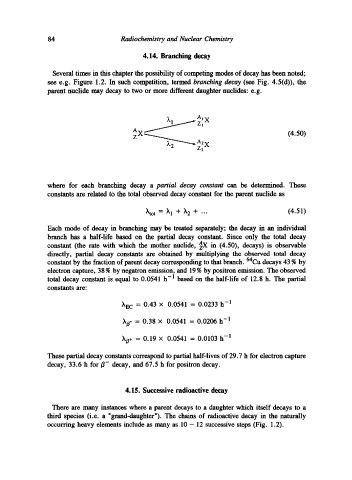
see e.g. Figure 1.2. In such competition, termed branching decay (see Fig. 4.5(d)), the
parent nuclide may decay to two or more different daughter nuclides: e.g.
Al x
A
(4.50)
Z2
where for each branching decay a partial decay constant can be determined. These
constants are related to the total observed decay constant for the parent nuclide as
~tot = ~kl + ~k2 + ... (4.51)
Each mode of decay in branching may be treated separately; the decay in an individual
branch has a half-life based on the partial decay constant. Since only the total decay
constant (the rate with which the mother nuclide, ~X in (4.50), decays) is observable
directly, partial decay constants are obtained by multiplying the observed total decay
constant by the fraction of parent decay corresponding to that branch. 64Cu decays 43 % by
electron capture, 38% by negatron emission, and 19% by positron emission. The observed
total decay constant is equal to 0.0541 h -1 based on the half-life of 12.8 h. The partial
constants are:
~kEC = 0.43 x 0.0541 = 0.0233 h -1
XB- = 0.38 x 0.0541 = 0.0206 h- l
h#+ = 0.19 • 0.0541 = 0.0103 h -1
These partial decay constants correspond to partial half-lives of 29.7 h for electron capture
decay, 33.6 h for B- decay, and 67.5 h for positron decay.
4.15. Successive radioactive decay
There are many instances where a parent decays to a daughter which itself decays to a
third species (i.e. a "grand-daughter"). The chains of radioactive decay in the naturally
occurring heavy elements include as many as 10 - 12 successive steps (Fig. 1.2).