Page 97 - Radiochemistry and nuclear chemistry
P. 97
86 Radiochemistry and Nuclear Chemistry
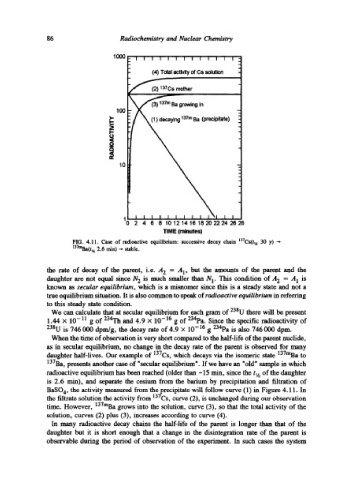
(4) Total activity of Cs solution
(2) 137Cs mother
137m Ba growing in
100
(1) decaying 137m Ba (precipitate)
10
10 2 4 6 8 10 12 14 16 18 20 22 24 26 28
TIME (minutes)
FIG. 4.11. Case of radioactive equilibrium: successive decay chain 137Cs(tt,~ 30 y) --
137mBa(tt,~ 2.6 min) -- stable.
the rate of decay of the parent, i.e. A 2 = A 1, but the amounts of the parent and the
daughter are not equal since N 2 is much smaller than N 1. This condition of A 2 = A 1 is
known as secular equilibrium, which is a misnomer since this is a steady state and not a
true equilibrium situation. It is also common to s~ of radioactive equilibrium in referring
to this steady state condition.
We can calculate that at secular equilibrium for each gram of 238U there will be present
1.44 x 10-tt g of 234Th and 4.9 x 10-16 g of 234pa. Since the specific radioactivity of
238U is 746 000 dpm/g, the decay rate of 4.9 • 10-16 g 234pa is also 746 000 dpm.
When the time of observation is very short compared to the half-life of the parent nuclide,
as in secular equilibrium, no change in the decay rate of the parent is observed for many
daughter half-lives. Our example of 137Cs, which decays via the isomeric state 137tuBa to
137Ba, presents another case of "secular equilibrium". If we have an "old" sample in which
radioactive equilibrium has been reached (older than -15 rain, since the tt/~ of the daughter
is 2.6 rain), and separate the cesium from the barium by precipitation and filtration of
BaSO 4, the activity measured from the precipitate will follow curve (1) in Figure 4.11. In
Cs,
the filtrate solution the activity from 137r cu e
rv (2), is unchanged during our observation
time. However, 137tuBa grows into the solution, curve (3), so that the total activity of the
solution, curves (2) plus (3), increases according to curve (4).
In many radioactive decay chains the half-life of the parent is longer than that of the
daughter but it is short enough that a change in the disintegration rate of the parent is
observable during the period of observation of the experiment. In such cases the system