Page 98 - Radiochemistry and nuclear chemistry
P. 98
Unstable Nuclei and Radioactive Decay 87
reaches the condition termed transient equilibrium. The length of time of observation of the
activity of the sample may be the determining factor as to whether it appears to be transient
or secular equilibrium. If a parent has a one month half-life and the observation of the
change in decay rates of parent and daughter extends over an hour or even a few days, the
data would follow the equation for secular equilibrium since the degree of change in the
parent decay would be negligible. However, if the observation extends over a period of
several weeks or months, then the change in the decay rate of the parent is significant and
it would appear as transient equilibrium.
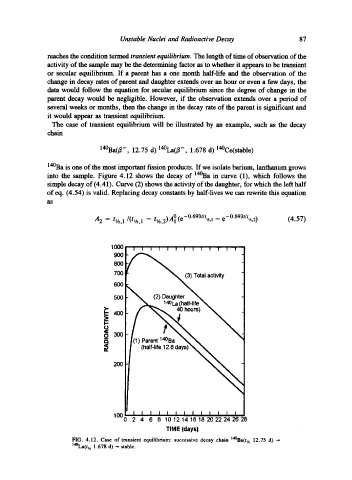
The case of transient equilibrium will be illustrated by an example, such as the decay
chain
14~ 12.75 d) 14~ 1.678 d) 14~
140Ba is one of the most important fission products. If we isolate barium, lanthanum grows
into the sample. Figure 4.12 shows the decay of 14~ in curve (1), which follows the
simple decay of (4.41). Curve (2) shows the activity of the daughter, for which the left half
of eq. (4.54) is valid. Replacing decay constants by half-lives we can rewrite this equation
as
A 2 = tl,~,l/(tl&,l - tl/:,2 ) A 0 (e-O.693t/t,~,, _ e-0.693t/,,~,2) (4.57)
1000 I I I I I I' I I I I I I I
900-
800
700 al activity
500 (2) Daughter ~ -
[ ~.~ 140 La (half-life
20O
1000 2 4 6 8 10121416182022242628
TIME (days)
FIG. 4.12. Case of transient equilibrium: successive decay chain t~(t,~ 12.75 d) ---
t'~...a(t,~ 1.678 d) ~ stable.