Page 133 - Reservoir Formation Damage
P. 133
Characterization of Reservoir Rock 115
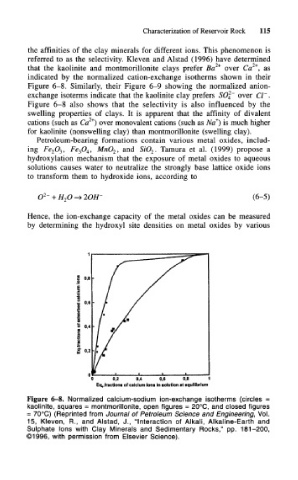
the affinities of the clay minerals for different ions. This phenomenon is
referred to as the selectivity. Kleven and Alstad (1996) have determined
2+
that the kaolinite and montmorillonite clays prefer Ba 2+ over Ca , as
indicated by the normalized cation-exchange isotherms shown in their
Figure 6-8. Similarly, their Figure 6-9 showing the normalized anion-
exchange isoterms indicate that the kaolinite clay prefers 5O|~ over Cl~.
Figure 6-8 also shows that the selectivity is also influenced by the
swelling properties of clays. It is apparent that the affinity of divalent
2+
+
cations (such as Ca ) over monovalent cations (such as Na ) is much higher
for kaolinite (nonswelling clay) than montmorillonite (swelling clay).
Petroleum-bearing formations contain various metal oxides, includ-
ing Fe 2O 3, Fe 3O 4, MnO 2, and SiO 2. Tamura et al. (1999) propose a
hydroxylation mechanism that the exposure of metal oxides to aqueous
solutions causes water to neutralize the strongly base lattice oxide ions
to transform them to hydroxide ions, according to
(6-5)
Hence, the ion-exchange capacity of the metal oxides can be measured
by determining the hydroxyl site densities on metal oxides by various
'0 0.2 0,4 0,6 0,8 1
Extractions of calcium ions In solution at equilibrium
Figure 6-8. Normalized calcium-sodium ion-exchange isotherms (circles =
kaolinite, squares = montmorillonite, open figures = 20°C, and closed figures
= 70°C) (Reprinted from Journal of Petroleum Science and Engineering, Vol.
15, Kleven, R., and Alstad, J., "Interaction of Alkali, Alkaline-Earth and
Sulphate Ions with Clay Minerals and Sedimentary Rocks," pp. 181-200,
©1996, with permission from Elsevier Science).