Page 134 - Reservoir Formation Damage
P. 134
116 Reservoir Formation Damage
0,2 0,4 0.6 0,8 1
Eq. fractions of sulphate Ions In solution
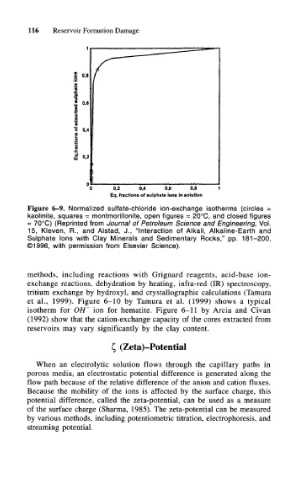
Figure 6-9. Normalized sulfate-chloride ion-exchange isotherms (circles =
kaolinite, squares = montmorillonite, open figures = 20°C, and closed figures
= 70°C) (Reprinted from Journal of Petroleum Science and Engineering, Vol.
15, Kleven, R., and Alstad, J., "Interaction of Alkali, Alkaline-Earth and
Sulphate Ions with Clay Minerals and Sedimentary Rocks," pp. 181-200,
©1996, with permission from Elsevier Science).
methods, including reactions with Grignard reagents, acid-base ion-
exchange reactions, dehydration by heating, infra-red (IR) spectroscopy,
tritium exchange by hydroxyl, and crystallographic calculations (Tamura
et al., 1999). Figure 6-10 by Tamura et al. (1999) shows a typical
isotherm for OH~ ion for hematite. Figure 6-11 by Arcia and Civan
(1992) show that the cation-exchange capacity of the cores extracted from
reservoirs may vary significantly by the clay content.
5 (Zeta)-Potential
When an electrolytic solution flows through the capillary paths in
porous media, an electrostatic potential difference is generated along the
flow path because of the relative difference of the anion and cation fluxes.
Because the mobility of the ions is affected by the surface charge, this
potential difference, called the zeta-potential, can be used as a measure
of the surface charge (Sharma, 1985). The zeta-potential can be measured
by various methods, including potentiometric titration, electrophoresis, and
streaming potential.