Page 185 - Reservoir Formation Damage
P. 185
Crystal Growth and Scale Formation in Porous Media 167
supersaturated solution. Whereas, secondary nucleation can occur at
relatively lower concentrations above the saturation line. The metastable
region represents the favorable conditions for crystal growth (Majors, 1999).
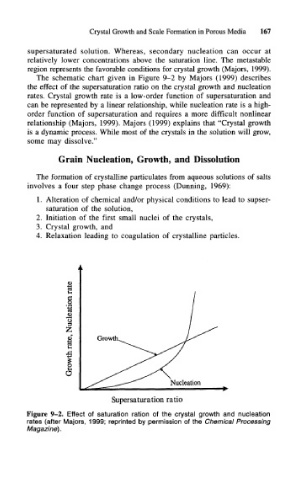
The schematic chart given in Figure 9-2 by Majors (1999) describes
the effect of the supersaturation ratio on the crystal growth and nucleation
rates. Crystal growth rate is a low-order function of supersaturation and
can be represented by a linear relationship, while nucleation rate is a high-
order function of supersaturation and requires a more difficult nonlinear
relationship (Majors, 1999). Majors (1999) explains that "Crystal growth
is a dynamic process. While most of the crystals in the solution will grow,
some may dissolve."
Grain Nucleation, Growth, and Dissolution
The formation of crystalline particulates from aqueous solutions of salts
involves a four step phase change process (Dunning, 1969):
1. Alteration of chemical and/or physical conditions to lead to supser-
saturation of the solution,
2. Initiation of the first small nuclei of the crystals,
3. Crystal growth, and
4. Relaxation leading to coagulation of crystalline particles.
I
Growth
1
Nucleation
Supersaturation ratio
Figure 9-2. Effect of saturation ration of the crystal growth and nucleation
rates (after Majors, 1999; reprinted by permission of the Chemical Processing
Magazine).