Page 362 - Reservoir Formation Damage
P. 362
342 Reservoir Formation Damage
affect of pH on the composition of the typical carbonate species, namely
2
'
H 2CO 3, HCO 3 and CO
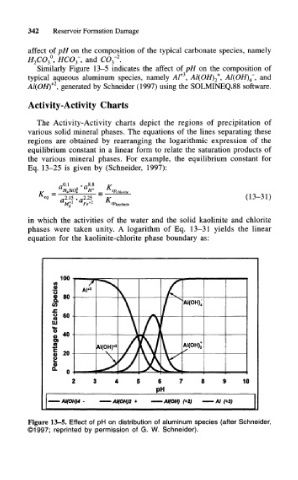
Similarly Figure 13-5 indicates the affect of pH on the composition of
+
3
typical aqueous aluminum species, namely At* , Al(OH) 2 , Al(OH) 4~, and
+
+2
Al(OH) , generated by Schneider (1997) using the SOLMINEQ.88 software.
Activity-Activity Charts
The Activity-Activity charts depict the regions of precipitation of
various solid mineral phases. The equations of the lines separating these
regions are obtained by rearranging the logarithmic expression of the
equilibrium constant in a linear form to relate the saturation products of
the various mineral phases. For example, the equilibrium constant for
Eq. 13-25 is given by (Schneider, 1997):
0.1
2.15 . ,,2.25 (13-31)
in which the activities of the water and the solid kaolinite and chlorite
phases were taken unity. A logarithm of Eq. 13-31 yields the linear
equation for the kaolinite-chlorite phase boundary as:
> AI(OH)4 - • M(OH)2 + •AJ(OH) (+2) • Al (+3)
Figure 13-5. Effect of pH on distribution of aluminum species (after Schneider,
©1997; reprinted by permission of G. W. Schneider).