Page 361 - Reservoir Formation Damage
P. 361
Inorganic Scaling and Geochemical Formation Damage 341
=[< +n ] -la"
p B (13-28)
I V)L} I (
Thus, a saturation index can be defined as:
'K^}
SI -log 10 (13-29)
and is used to determine the state of saturation of an aqueous solution
by a mineral as follows:
< 0 , undersaturated
SI = 0 , saturated
(13-30)
> 0 , supersaturated
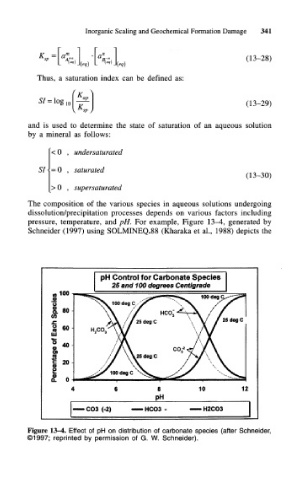
The composition of the various species in aqueous solutions undergoing
dissolution/precipitation processes depends on various factors including
pressure, temperature, and pH. For example, Figure 13-4, generated by
Schneider (1997) using SOLMINEQ.88 (Kharaka et al., 1988) depicts the
pH Control for Carbonate Species
25 and 100 degrees Centigrade
100
COS (-2) HCO3 - H2CO3
Figure 13-4. Effect of pH on distribution of carbonate species (after Schneider,
©1997; reprinted by permission of G. W. Schneider).