Page 279 - Theory and Problems of BEGINNING CHEMISTRY
P. 279
268 ORGANIC CHEMISTRY [CHAP. 18
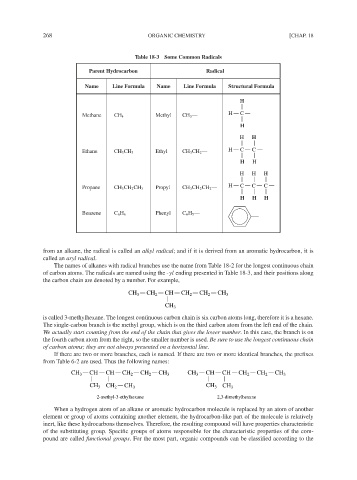
Table 18-3 Some Common Radicals
Parent Hydrocarbon Radical
Name Line Formula Name Line Formula Structural Formula
H
Methane CH 4 Methyl CH 3 — H C
H
H H
Ethane CH 3 CH 3 Ethyl CH 3 CH 2 — H C C
H H
H H H
Propane CH 3 CH 2 CH 3 Propyl CH 3 CH 2 CH 2 — H C C C
H H H
Benzene C 6 H 6 Phenyl C 6 H 5 —
from an alkane, the radical is called an alkyl radical; and if it is derived from an aromatic hydrocarbon, it is
called an aryl radical.
The names of alkanes with radical branches use the name from Table 18-2 for the longest continuous chain
of carbon atoms. The radicals are named using the -yl ending presented in Table 18-3, and their positions along
the carbon chain are denoted by a number. For example,
CH 3 CH 2 CH CH 2 CH 2 CH 3
CH 3
is called 3-methylhexane. The longest continuous carbon chain is six carbon atoms long, therefore it is a hexane.
The single-carbon branch is the methyl group, which is on the third carbon atom from the left end of the chain.
We actually start counting from the end of the chain that gives the lower number. In this case, the branch is on
the fourth carbon atom from the right, so the smaller number is used. Be sure to use the longest continuous chain
of carbon atoms; they are not always presented on a horizontal line.
If there are two or more branches, each is named. If there are two or more identical branches, the prefixes
from Table 6-2 are used. Thus the following names:
CH 3 CH CH CH 2 CH 2 CH 3 CH 3 CH CH CH 2 CH 2 CH 3
CH 3 CH 2 CH 3 CH 3 CH 3
2-methyl-3-ethylhexane 2,3-dimethylhexane
When a hydrogen atom of an alkane or aromatic hydrocarbon molecule is replaced by an atom of another
element or group of atoms containing another element, the hydrocarbon-like part of the molecule is relatively
inert, like these hydrocarbons themselves. Therefore, the resulting compound will have properties characteristic
of the substituting group. Specific groups of atoms responsible for the characteristic properties of the com-
pound are called functional groups. For the most part, organic compounds can be classified according to the