Page 158 - Science at the nanoscale
P. 158
10:15
RPS: PSP0007 - Science-at-Nanoscale
June 5, 2009
Formation and Self-Assembly at the Nanoscale
148
super
saturated
insoluble
solutes
nuclei
nanoparticles
nucleation
growth
concentration
nucleation concentration
saturation concentration
solute
time
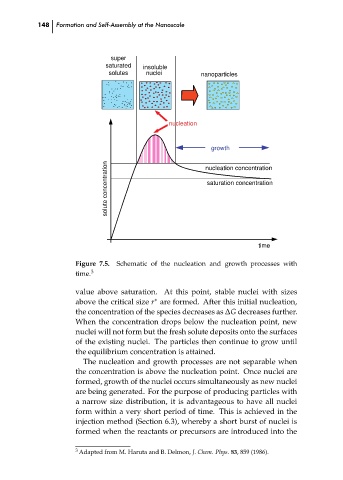
Schematic of the nucleation and growth processes with
Figure 7.5.
3
time.
value above saturation. At this point, stable nuclei with sizes
∗
above the critical size r are formed. After this initial nucleation,
the concentration of the species decreases as ∆G decreases further.
When the concentration drops below the nucleation point, new
nuclei will not form but the fresh solute deposits onto the surfaces
of the existing nuclei. The particles then continue to grow until ch07
the equilibrium concentration is attained.
The nucleation and growth processes are not separable when
the concentration is above the nucleation point. Once nuclei are
formed, growth of the nuclei occurs simultaneously as new nuclei
are being generated. For the purpose of producing particles with
a narrow size distribution, it is advantageous to have all nuclei
form within a very short period of time. This is achieved in the
injection method (Section 6.3), whereby a short burst of nuclei is
formed when the reactants or precursors are introduced into the
3 Adapted from M. Haruta and B. Delmon, J. Chem. Phys. 83, 859 (1986).