Page 218 - Science at the nanoscale
P. 218
10:17
RPS: PSP0007 - Science-at-Nanoscale
June 5, 2009
Future Trends
208
The concept of molecular electronics has created much excite-
ment among scientists and technologists due to the prospect of
size reduction in electronics offered by the molecular-level con-
trol of properties. Although the original molecular rectifier was
predicted as far back as 1974, a commercialisable single molecule
device has yet to be demonstrated.
The past few decades has seen the discovery of fascinating new
allotropes of carbon. The fullerenes, discovered in 1985 by Robert
Curl, Harold Kroto and Richard Smalley, are a family of car-
bon allotropes named after Richard Buckminster Fuller and are
sometimes also called buckyballs. Kroto, Curl, and Smalley were
awarded the 1996 Nobel Prize in Chemistry for their discovery
of this class of compounds. Fullerenes are molecules composed
entirely of carbon, in the form of a hollow sphere, ellipsoid, or
tube. Cylindrical fullerenes are called carbon nanotubes. Fullerenes
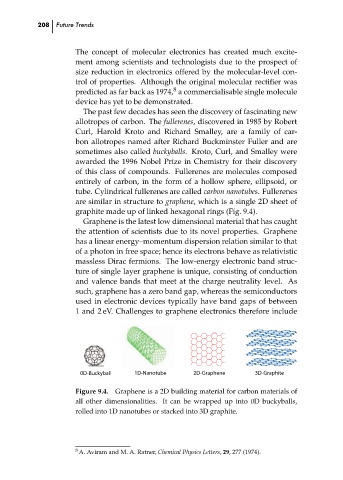
are similar in structure to graphene, which is a single 2D sheet of
graphite made up of linked hexagonal rings (Fig. 9.4).
Graphene is the latest low dimensional material that has caught
the attention of scientists due to its novel properties. Graphene
has a linear energy–momentum dispersion relation similar to that
of a photon in free space; hence its electrons behave as relativistic
massless Dirac fermions. The low-energy electronic band struc-
ture of single layer graphene is unique, consisting of conduction
and valence bands that meet at the charge neutrality level. As
such, graphene has a zero band gap, whereas the semiconductors
used in electronic devices typically have band gaps of between
1 and 2 eV. Challenges to graphene electronics therefore include
0D-Buckyball 1D-Nanotube 8 2D-Graphene 3D-Graphite ch09
Figure 9.4. Graphene is a 2D building material for carbon materials of
all other dimensionalities. It can be wrapped up into 0D buckyballs,
rolled into 1D nanotubes or stacked into 3D graphite.
8 A. Aviram and M. A. Ratner, Chemical Physics Letters, 29, 277 (1974).