Page 249 - Semiconductor Manufacturing Handbook
P. 249
Geng(SMH)_CH17.qxd 04/04/2005 19:57 Page 17.4
CHEMICAL MECHANICAL POLISHING
17.4 WAFER PROCESSING
2.2
1.8 −6 0
WO 5 2−
1.4
1.0 WO 3 WO 4 2− Select Refs
Electric potential V −0.2 W O WO 2 −0.45
0.6
E(V)
0.2
2
5
pH
7.18
pO (bar)
−0.6
2
0.0 E−89
−1.0 pH 2 (bar)
W 9.1 E+0
−1.4
−1.8
−2 0 2 4 6 8 10 12 14 16
pH
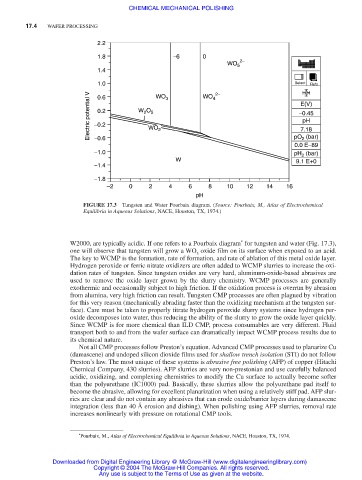
FIGURE 17.3 Tungsten and Water Pourbaix diagram. (Source: Pourbaix, M., Atlas of Electrochemical
Equilibria in Aqueous Solutions, NACE, Houston, TX, 1974.)
*
W2000, are typically acidic. If one refers to a Pourbaix diagram for tungsten and water (Fig. 17.3),
one will observe that tungsten will grow a WO oxide film on its surface when exposed to an acid.
3
The key to WCMP is the formation, rate of formation, and rate of ablation of this metal oxide layer.
Hydrogen peroxide or ferric nitrate oxidizers are often added to WCMP slurries to increase the oxi-
dation rates of tungsten. Since tungsten oxides are very hard, aluminum-oxide-based abrasives are
used to remove the oxide layer grown by the slurry chemistry. WCMP processes are generally
exothermic and occasionally subject to high friction. If the oxidation process is overrun by abrasion
from alumina, very high friction can result. Tungsten CMP processes are often plagued by vibration
for this very reason (mechanically abrading faster than the oxidizing mechanism at the tungsten sur-
face). Care must be taken to properly titrate hydrogen peroxide slurry systems since hydrogen per-
oxide decomposes into water, thus reducing the ability of the slurry to grow the oxide layer quickly.
Since WCMP is for more chemical than ILD CMP, process consumables are very different. Fluid
transport both to and from the wafer surface can dramatically impact WCMP process results due to
its chemical nature.
Not all CMP processes follow Preston’s equation. Advanced CMP processes used to planarize Cu
(damascene) and undoped silicon dioxide films used for shallow trench isolation (STI) do not follow
Preston’s law. The most unique of these systems is abrasive free polishing (AFP) of copper (Hitachi
Chemical Company, 430 slurries). AFP slurries are very non-prestonian and use carefully balanced
acidic, oxidizing, and complexing chemistries to modify the Cu surface to actually become softer
than the polyurethane (IC1000) pad. Basically, these slurries allow the polyurethane pad itself to
become the abrasive, allowing for excellent planarization when using a relatively stiff pad. AFP slur-
ries are clear and do not contain any abrasives that can erode oxide/barrier layers during damascene
integration (less than 40 Å erosion and dishing). When polishing using AFP slurries, removal rate
increases nonlinearly with pressure on rotational CMP tools.
* Pourbaix, M., Atlas of Electrochemical Equilibria in Aqueous Solutions, NACE, Houston, TX, 1974.
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2004 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.