Page 254 - Soil and water contamination, 2nd edition
P. 254
Chemical transformation 241
0
5
10
15
Depth
20
25
30
6642 6642 6642 35
0 50 100 150 200 250 300 350
Horizontal distance
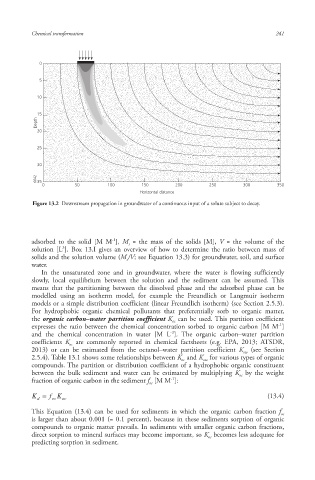
Figure 13.2 Downstream propagation in groundwater of a continuous input of a solute subject to decay.
-1
adsorbed to the solid [M M ], M = the mass of the solids [M], V = the volume of the
s
3
solution [L ]. Box 13.I gives an overview of how to determine the ratio between mass of
solids and the solution volume (M /V; see Equation 13.3) for groundwater, soil, and surface
s
water.
In the unsaturated zone and in groundwater, where the water is flowing sufficiently
slowly, local equilibrium between the solution and the sediment can be assumed. This
means that the partitioning between the dissolved phase and the adsorbed phase can be
modelled using an isotherm model, for example the Freundlich or Langmuir isotherm
models or a simple distribution coefficient (linear Freundlich isotherm ) (see Section 2.5.3).
For hydrophobic organic chemical pollutants that preferentially sorb to organic matter ,
the organic carbon–water partition coefficient K can be used. This partition coefficient
oc
-1
expresses the ratio between the chemical concentration sorbed to organic carbon [M M ]
-3
and the chemical concentration in water [M L ]. The organic carbon–water partition
coefficient s K are commonly reported in chemical factsheets (e.g. EPA, 2013; ATSDR,
oc
2013) or can be estimated from the octanol–water partition coefficient K (see Section
ow
2.5.4). Table 13.1 shows some relationships between K and K for various types of organic
oc ow
compounds. The partition or distribution coefficient of a hydrophobic organic constituent
between the bulk sediment and water can be estimated by multiplying K by the weight
oc
-1
fraction of organic carbon in the sediment f [M M ]:
oc
K f oc K oc (13.4)
d
This Equation (13.4) can be used for sediments in which the organic carbon fraction f
oc
is larger than about 0.001 (= 0.1 percent), because in these sediments sorption of organic
compounds to organic matter prevails. In sediments with smaller organic carbon fractions,
direct sorption to mineral surfaces may become important, so K becomes less adequate for
oc
predicting sorption in sediment.
10/1/2013 6:45:10 PM
Soil and Water.indd 253
Soil and Water.indd 253 10/1/2013 6:45:10 PM