Page 46 - Strategies and Applications in Quantum Chemistry From Molecular Astrophysics to Molecular Engineer
P. 46
THEORY OF ORBITAL OPTIMIZATION IN SCF AND MCSF CALCULATIONS 31
Thus we will use the result of calculations of the wave function of expanded in a
gaussian basis to provide numerical tests of the qualitative discussion on the orbital
optimisation theory presented in the above sections 2 and 3.
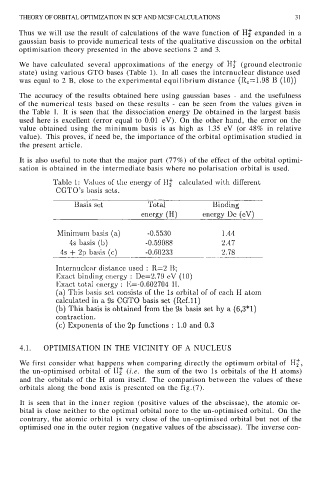
We have calculated several approximations of the energy of (ground electronic
state) using various GTO bases (Table 1). In all cases the intcrnuclear distance used
was equal to 2 B, close to the experimental equilibrium distance
The accuracy of the results obtained here using gaussian bases - and the usefulness
of the numerical tests based on these results - can be seen from the values given in
the Table 1. It is seen that the dissociation energy De obtained in the largest basis
used here is excellent (error equal to 0.01 eV). On the other hand, the error on the
value obtained using the minimum basis is as high as 1.35 eV (or 48% in relative
value). This proves, if need be, the importance of the orbital optimisation studied in
the present article.
It is also useful to note that the major part (77%) of the effect of the orbital optimi-
sation is obtained in the intermediate basis where no polarisation orbital is used.
4.1. OPTIMISATION IN THE VICINITY OF A NUCLEUS
We first consider what happens when comparing directly the optimum orbital of
the un-optimised orbital of (i.e. the sum of the two 1s orbitals of the H atoms)
and the orbitals of the H atom itself. The comparison between the values of these
orbitals along the bond axis is presented on the fig.(7).
It is seen that in the inner region (positive values of the abscissae), the atomic or-
bital is close neither to the optimal orbital nore to the un-optimised orbital. On the
contrary, the atomic orbital is very close of the un-optimised orbital but not of the
optimised one in the outer region (negative values of the abscissae). The inverse con-