Page 48 - Strategies and Applications in Quantum Chemistry From Molecular Astrophysics to Molecular Engineer
P. 48
THEORY OF ORBITAL OPTIMIZATION IN SCF AND MCSF CALCULATIONS 33
be close of any atomic-like orbital.
4.2. OPTIMISATION IN THE ASYMPTOTIC REGION
When expanding the orbital in partial waves with origin at the midpoint of the
molecule (center of charge of the molecule-minus-one-electron) the p wave vanishes,
and only the s wave has to be considered. According to Sec.3, this partial wave must
be proportionnal to the irregular solution of the hydrogen-like system with atomic
number Z’= 2 and with e equal to the exact orbital energy (-1.102 H).
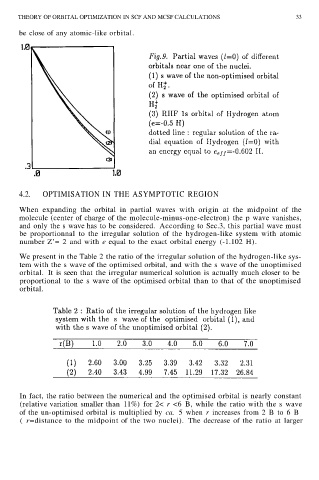
We present in the Table 2 the ratio of the irregular solution of the hydrogen-like sys-
tem with the s wave of the optimised orbital, and with the s wave of the unoptimised
orbital. It is seen that the irregular numerical solution is actually much closer to be
proportional to the s wave of the optimised orbital than to that of the unoptimised
orbital.
In fact, the ratio between the numerical and the optimised orbital is nearly constant
(relative variation smaller than 11%) for 2< r <6 B, while the ratio with the s wave
of the un-optimised orbital is multiplied by ca. 5 when r increases from 2 B to 6 B
( r=distance to the midpoint of the two nuclei). The decrease of the ratio at larger