Page 66 - Synthetic Fuels Handbook
P. 66
54 CHAPTER TWO
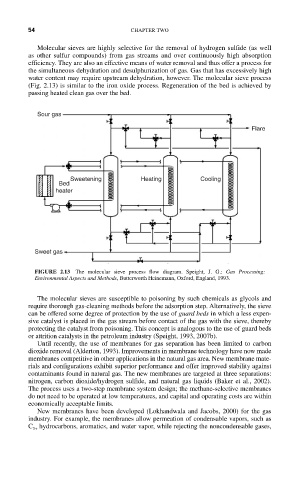
Molecular sieves are highly selective for the removal of hydrogen sulfide (as well
as other sulfur compounds) from gas streams and over continuously high absorption
efficiency. They are also an effective means of water removal and thus offer a process for
the simultaneous dehydration and desulphurization of gas. Gas that has excessively high
water content may require upstream dehydration, however. The molecular sieve process
(Fig. 2.13) is similar to the iron oxide process. Regeneration of the bed is achieved by
passing heated clean gas over the bed.
Sour gas
Flare
Sweetening Heating Cooling
Bed
heater
Sweet gas
FIGURE 2.13 The molecular sieve process flow diagram. Speight, J. G.: Gas Processing:
Environmental Aspects and Methods, Butterworth Heinemann, Oxford, England, 1993.
The molecular sieves are susceptible to poisoning by such chemicals as glycols and
require thorough gas-cleaning methods before the adsorption step. Alternatively, the sieve
can be offered some degree of protection by the use of guard beds in which a less expen-
sive catalyst is placed in the gas stream before contact of the gas with the sieve, thereby
protecting the catalyst from poisoning. This concept is analogous to the use of guard beds
or attrition catalysts in the petroleum industry (Speight, 1993, 2007b).
Until recently, the use of membranes for gas separation has been limited to carbon
dioxide removal (Alderton, 1993). Improvements in membrane technology have now made
membranes competitive in other applications in the natural gas area. New membrane mate-
rials and configurations exhibit superior performance and offer improved stability against
contaminants found in natural gas. The new membranes are targeted at three separations:
nitrogen, carbon dioxide/hydrogen sulfide, and natural gas liquids (Baker et al., 2002).
The process uses a two-step membrane system design; the methane-selective membranes
do not need to be operated at low temperatures, and capital and operating costs are within
economically acceptable limits.
New membranes have been developed (Lokhandwala and Jacobs, 2000) for the gas
industry. For example, the membranes allow permeation of condensable vapors, such as
C hydrocarbons, aromatics, and water vapor, while rejecting the noncondensable gases,
3+