Page 96 - The Biochemistry of Inorganic Polyphosphates
P. 96
WU095/Kulaev
WU095-06
Enzymes of polyphosphate biosynthesis and degradation
80 March 9, 2004 15:32 Char Count= 0
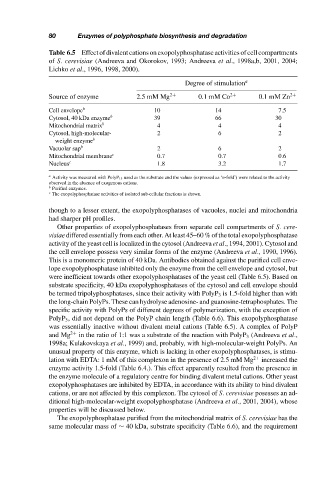
Table 6.5 Effect of divalent cations on exopolyphosphatase activities of cell compartments
of S. cerevisiae (Andreeva and Okorokov, 1993; Andreeva et al., 1998a,b, 2001, 2004;
Lichko et al., 1996, 1998, 2000).
Degree of stimulation a
Source of enzyme 2.5 mM Mg 2+ 0.1 mM Co 2+ 0.1 mM Zn 2+
Cell envelope b 10 14 7.5
Cytosol, 40 kDa enzyme b 39 66 30
Mitochondrial matrix b 4 4 4
Cytosol, high-molecular- 2 6 2
weight enzyme b
Vacuolar sap b 2 6 2
Mitochondrial membrane c 0.7 0.7 0.6
Nucleus c 1.8 3.2 1.7
a Activity was measured with PolyP 15 used as the substrate and the values (expressed as ‘n-fold’) were related to the activity
observed in the absence of exogenous cations.
b Purified enzymes.
c The exopolyphosphatase activities of isolated sub-cellular fractions is shown.
though to a lesser extent, the exopolyphosphatases of vacuoles, nuclei and mitochondria
had sharper pH profiles.
Other properties of exopolyphosphatases from separate cell compartments of S. cere-
visiae differed essentially from each other. At least 45–60 % of the total exopolyphosphatase
activity of the yeast cell is localized in the cytosol (Andreeva et al., 1994, 2001). Cytosol and
the cell envelope possess very similar forms of the enzyme (Andreeva et al., 1990, 1996).
This is a monomeric protein of 40 kDa. Antibodies obtained against the purified cell enve-
lope exopolyphosphatase inhibited only the enzyme from the cell envelope and cytosol, but
were inefficient towards other exopolyphosphatases of the yeast cell (Table 6.5). Based on
substrate specificity, 40 kDa exopolyphosphatases of the cytosol and cell envelope should
be termed tripolyphosphatases, since their activity with PolyP 3 is 1.5-fold higher than with
the long-chain PolyPs. These can hydrolyse adenosine- and guanosine-tetraphosphates. The
specific activity with PolyPs of different degrees of polymerization, with the exception of
PolyP 3 , did not depend on the PolyP chain length (Table 6.6). This exopolyphosphatase
was essentially inactive without divalent metal cations (Table 6.5). A complex of PolyP
and Mg 2+ in the ratio of 1:1 was a substrate of the reaction with PolyP 3 (Andreeva et al.,
1998a; Kulakovskaya et al., 1999) and, probably, with high-molecular-weight PolyPs. An
unusual property of this enzyme, which is lacking in other exopolyphosphatases, is stimu-
lation with EDTA: 1 mM of this complexon in the presence of 2.5 mM Mg 2+ increased the
enzyme activity 1.5-fold (Table 6.4.). This effect apparently resulted from the presence in
the enzyme molecule of a regulatory centre for binding divalent metal cations. Other yeast
exopolyphosphatases are inhibited by EDTA, in accordance with its ability to bind divalent
cations, or are not affected by this complexon. The cytosol of S. cerevisiae posesses an ad-
ditional high-molecular-weight exopolyphosphatase (Andreeva et al., 2001, 2004), whose
properties will be discussed below.
The exopolyphosphatase purified from the mitochondrial matrix of S. cerevisiae has the
same molecular mass of ∼ 40 kDa, substrate specificity (Table 6.6), and the requirement