Page 97 - The Biochemistry of Inorganic Polyphosphates
P. 97
Char Count= 0
March 9, 2004
15:32
WU095/Kulaev
WU095-06
Enzymes of polyphosphate degradation 81
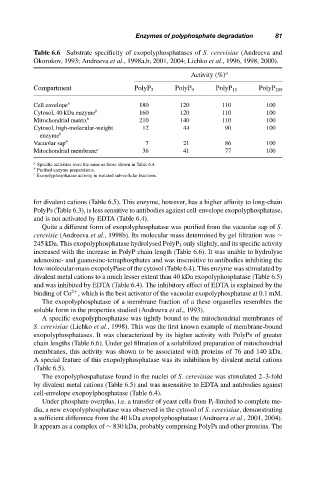
Table 6.6 Substrate specificity of exopolyphosphatases of S. cerevisiae (Andreeva and
Okorokov, 1993; Andreeva et al., 1998a,b, 2001, 2004; Lichko et al., 1996, 1998, 2000).
Activity (%) a
Compartment PolyP 3 PolyP 9 PolyP 15 PolyP 208
Cell envelope b 180 120 110 100
Cytosol, 40 kDa enzyme b 160 120 110 100
Mitochondrial matrix b 210 140 110 100
Cytosol, high-molecular-weight 12 44 90 100
enzyme b
Vacuolar sap b 7 21 86 100
Mitochondrial membrane c 36 41 77 100
a
Specific activities were the same as those shown in Table 6.4.
b
Purified enzyme preparations.
c
Exopolyphosphatase activity in isolated sub-cellular fractions.
for divalent cations (Table 6.5). This enzyme, however, has a higher affinity to long-chain
PolyPs (Table 6.3), is less sensitive to antibodies against cell-envelope exopolyphosphatase,
and is not activated by EDTA (Table 6.4).
Quite a different form of exopolyphosphatase was purified from the vacuolar sap of S.
cerevisie (Andreeva et al., 1998b). Its molecular mass determined by gel filtration was ∼
245 kDa. This exopolyphosphatase hydrolysed PolyP 3 only slightly, and its specific activity
increased with the increase in PolyP chain length (Table 6.6). It was unable to hydrolyse
adenosine- and guanosine-tetraphosphates and was insensitive to antibodies inhibiting the
low-molecular-mass exopolyPase of the cytosol (Table 6.4). This enzyme was stimulated by
divalent metal cations to a much lesser extent than 40 kDa exopolyphosphatase (Table 6.5)
and was inhibited by EDTA (Table 6.4). The inhibitory effect of EDTA is explained by the
binding of Co , which is the best activator of the vacuolar exopolyphosphatase at 0.1 mM.
2+
The exopolyphosphatase of a membrane fraction of a these organelles resembles the
soluble form in the properties studied (Andreeva et al., 1993).
A specific exopolyphosphatase was tightly bound to the mitochondrial membranes of
S. cerevisiae (Lichko et al., 1998). This was the first known example of membrane-bound
exopolyphosphatases. It was characterized by its higher activity with PolyPs of greater
chain lengths (Table 6.6). Under gel filtration of a solubilized preparation of mitochondrial
membranes, this activity was shown to be associated with proteins of 76 and 140 kDa.
A special feature of this exopolyphosphatase was its inhibition by divalent metal cations
(Table 6.5).
The exopolyphospahatase found in the nuclei of S. cerevisiae was stimulated 2–3-fold
by divalent metal cations (Table 6.5) and was insensitive to EDTA and antibodies against
cell-envelope exopoylphosphatase (Table 6.4).
Under phosphate overplus, i.e. a transfer of yeast cells from P i -limited to complete me-
dia, a new exopolyphosphatase was observed in the cytosol of S. cerevisiae, demonstrating
a sufficient difference from the 40 kDa exopolyphosphatase (Andreeva et al., 2001, 2004).
It appears as a complex of ∼ 830 kDa, probably comprising PolyPs and other proteins. The