Page 99 - The Biochemistry of Inorganic Polyphosphates
P. 99
15:32
March 9, 2004
Char Count= 0
WU095-06
WU095/Kulaev
Enzymes of polyphosphate degradation 83
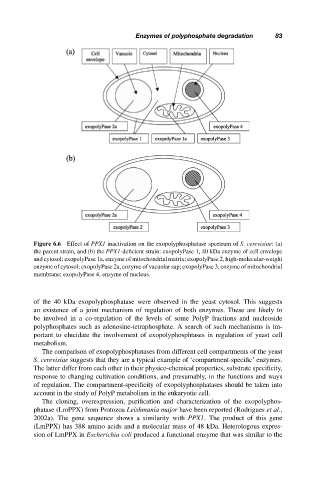
Figure 6.6 Effect of PPX1 inactivation on the exopolyphosphatase spcetrum of S. cerevisiae: (a)
the parent strain, and (b) the PPX1-deficient strain: exopolyPase 1, 40 kDa enzyme of cell envelope
and cytosol; exopolyPase 1a, enzyme of mitochondrial matrix; exopolyPase 2, high-molecular-weight
enzyme of cytosol; exopolyPase 2a, enzyme of vacuolar sap; exopolyPase 3, enzyme of mitochondrial
membrane; exopolyPase 4, enzyme of nucleus.
of the 40 kDa exopolyphosphatase were observed in the yeast cytosol. This suggests
an existence of a joint mechanism of regulation of both enzymes. These are likely to
be involved in a co-regulation of the levels of some PolyP fractions and nucleoside
polyphosphates such as adenosine-tetraphosphate. A search of such mechanisms is im-
portant to elucidate the involvement of exopolyphosphtases in regulation of yeast cell
metabolism.
The comparison of exopolyphosphatases from different cell compartments of the yeast
S. cerevisiae suggests that they are a typical example of ‘compartment-specific’ enzymes.
The latter differ from each other in their physico-chemical properties, substrate specificity,
response to changing cultivation conditions, and presumably, in the functions and ways
of regulation. The compartment-specificity of exopolyphosphatases should be taken into
account in the study of PolyP metabolism in the eukaryotic cell.
The cloning, overexpression, purification and characterization of the exopolyphos-
phatase (LmPPX) from Protozoa Leishmania major have been reported (Rodrigues et al.,
2002a). The gene sequence shows a similarity with PPX1. The product of this gene
(LmPPX) has 388 amino acids and a molecular mass of 48 kDa. Heterologous expres-
sion of LmPPX in Escherichia coli produced a functional enzyme that was similar to the