Page 21 - Welding of Aluminium and its Alloys
P. 21
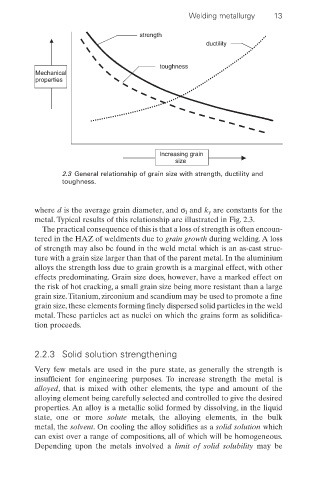
Welding metallurgy 13
strength
ductility
toughness
Mechanical
properties
Increasing grain
size
2.3 General relationship of grain size with strength, ductility and
toughness.
where d is the average grain diameter, and s I and k y are constants for the
metal. Typical results of this relationship are illustrated in Fig. 2.3.
The practical consequence of this is that a loss of strength is often encoun-
tered in the HAZ of weldments due to grain growth during welding. A loss
of strength may also be found in the weld metal which is an as-cast struc-
ture with a grain size larger than that of the parent metal. In the aluminium
alloys the strength loss due to grain growth is a marginal effect, with other
effects predominating. Grain size does, however, have a marked effect on
the risk of hot cracking, a small grain size being more resistant than a large
grain size.Titanium, zirconium and scandium may be used to promote a fine
grain size, these elements forming finely dispersed solid particles in the weld
metal. These particles act as nuclei on which the grains form as solidifica-
tion proceeds.
2.2.3 Solid solution strengthening
Very few metals are used in the pure state, as generally the strength is
insufficient for engineering purposes. To increase strength the metal is
alloyed, that is mixed with other elements, the type and amount of the
alloying element being carefully selected and controlled to give the desired
properties. An alloy is a metallic solid formed by dissolving, in the liquid
state, one or more solute metals, the alloying elements, in the bulk
metal, the solvent. On cooling the alloy solidifies as a solid solution which
can exist over a range of compositions, all of which will be homogeneous.
Depending upon the metals involved a limit of solid solubility may be