Page 25 - Welding of Aluminium and its Alloys
P. 25
Welding metallurgy 17
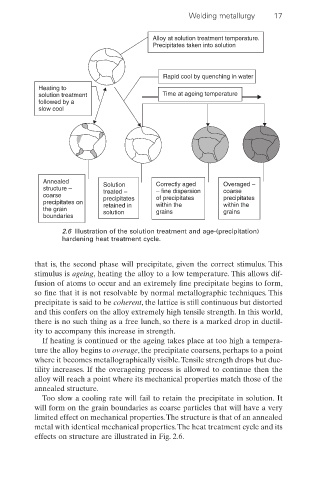
Alloy at solution treatment temperature.
Precipitates taken into solution
Rapid cool by quenching in water
Heating to
solution treatment Time at ageing temperature
followed by a
slow cool
Annealed Solution Correctly aged Overaged –
structure – treated – – fine dispersion coarse
coarse precipitates of precipitates precipitates
precipitates on retained in within the within the
the grain solution grains grains
boundaries
2.6 Illustration of the solution treatment and age-(precipitation)
hardening heat treatment cycle.
that is, the second phase will precipitate, given the correct stimulus. This
stimulus is ageing, heating the alloy to a low temperature. This allows dif-
fusion of atoms to occur and an extremely fine precipitate begins to form,
so fine that it is not resolvable by normal metallographic techniques. This
precipitate is said to be coherent, the lattice is still continuous but distorted
and this confers on the alloy extremely high tensile strength. In this world,
there is no such thing as a free lunch, so there is a marked drop in ductil-
ity to accompany this increase in strength.
If heating is continued or the ageing takes place at too high a tempera-
ture the alloy begins to overage, the precipitate coarsens, perhaps to a point
where it becomes metallographically visible.Tensile strength drops but duc-
tility increases. If the overageing process is allowed to continue then the
alloy will reach a point where its mechanical properties match those of the
annealed structure.
Too slow a cooling rate will fail to retain the precipitate in solution. It
will form on the grain boundaries as coarse particles that will have a very
limited effect on mechanical properties.The structure is that of an annealed
metal with identical mechanical properties.The heat treatment cycle and its
effects on structure are illustrated in Fig. 2.6.