Page 22 - Welding of Aluminium and its Alloys
P. 22
14 The welding of aluminium and its alloys
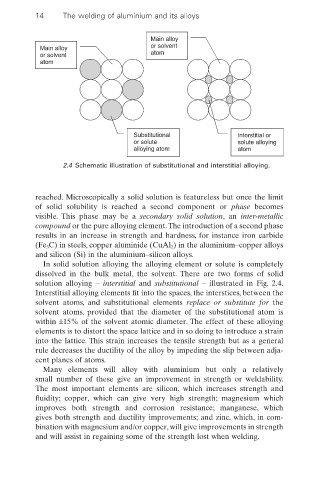
Main alloy
Main alloy or solvent
or solvent atom
atom
Substitutional Interstitial or
or solute solute alloying
alloying atom atom
2.4 Schematic illustration of substitutional and interstitial alloying.
reached. Microscopically a solid solution is featureless but once the limit
of solid solubility is reached a second component or phase becomes
visible. This phase may be a secondary solid solution,an inter-metallic
compound or the pure alloying element.The introduction of a second phase
results in an increase in strength and hardness, for instance iron carbide
(Fe 3C) in steels, copper aluminide (CuAl 2) in the aluminium–copper alloys
and silicon (Si) in the aluminium–silicon alloys.
In solid solution alloying the alloying element or solute is completely
dissolved in the bulk metal, the solvent. There are two forms of solid
solution alloying – interstitial and substitutional – illustrated in Fig. 2.4.
Interstitial alloying elements fit into the spaces, the interstices, between the
solvent atoms, and substitutional elements replace or substitute for the
solvent atoms, provided that the diameter of the substitutional atom is
within ±15% of the solvent atomic diameter. The effect of these alloying
elements is to distort the space lattice and in so doing to introduce a strain
into the lattice. This strain increases the tensile strength but as a general
rule decreases the ductility of the alloy by impeding the slip between adja-
cent planes of atoms.
Many elements will alloy with aluminium but only a relatively
small number of these give an improvement in strength or weldability.
The most important elements are silicon, which increases strength and
fluidity; copper, which can give very high strength; magnesium which
improves both strength and corrosion resistance; manganese, which
gives both strength and ductility improvements; and zinc, which, in com-
bination with magnesium and/or copper, will give improvements in strength
and will assist in regaining some of the strength lost when welding.