Page 27 - The engineering of chemical reactions
P. 27
M o d e l i n g 1 1
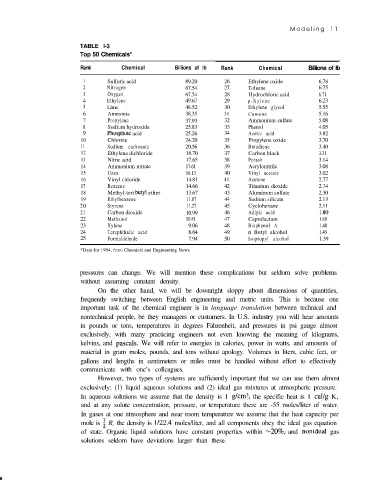
TABLE l-3
Top 50 Chemicals*
Rank Chemical Billions of lb Rank Chemical Billions of lb
1 Sulfuric acid 89.20 26 Ethylene oxide 6.78
2 Nitrogen 67.54 27 Toluene 6.75
3 Oxygen 67.54 28 Hydrochloric acid 6.71
4 Ethylene 49.67 29 p-Xylene 6.23
5 Lime 48.52 30 Ethylene glycol 5.55
6 Ammonia 38.35 31 Cumene 5.16
7 Propylene 37.93 32 Ammonium sulfate 5.08
8 Sodium hydroxide 25.83 33 Phenol 4.05
9 Phosphoic acid 25.26 34 Acetic acid 3.82
10 Chlorine 24.20 35 Propylene oxide 3.70
11 Sodium carbonate 20.56 36 Butadiene 3.40
12 Ethylene dichloride 18.70 37 Carbon black 3.31
13 Nitric acid 17.65 38 Potash 3.14
14 Ammonium nitrate 17.61 39 Acrylonitrile 3.08
15 Urea 16.13 40 Vinyl acetate 3.02
16 Vinyl chloride 14.81 41 Acetone 2.77
17 Benzene 14.66 42 Titanium dioxide 2.74
18 Methyl-tert butyl ether 13.67 43 Aluminum sulfate 2.30
19 Ethylbenzene 11.87 44 Sodium silicate 2.13
20 Styrene 11.27 45 Cyclohexane 2.11
21 Carbon dioxide 10.99 46 Adipic acid 1 .X0
22 Methanol 10.81 47 Caprolactam 1.68
23 Xylene 9.06 48 Bisphenol A 1.48
24 Terephthalic acid 8.64 49 n Butyl alcohol 1.45
25 Formaldehyde 7.94 50 Isopropyl alcohol 1.39
*Data for 1994, from Chemical and Engineering News
pressures can change. We will mention these complications but seldom solve problems
without assuming constant density.
On the other hand, we will be downright sloppy about dimensions of quantities,
frequently switching between English engineering and metric units. This is because one
important task of the chemical engineer is in language translation between technical and
nontechnical people, be they managers or customers. In U.S. industry you will hear amounts
in pounds or tons, temperatures in degrees Fahrenheit, and pressures in psi gauge almost
exclusively, with many practicing engineers not even knowing the meaning of kilograms,
kelvins, and pascals. We will refer to energies in calories, power in watts, and amounts of
material in gram moles, pounds, and tons without apology. Volumes in liters, cubic feet, or
gallons and lengths in centimeters or miles must be handled without effort to effectively
communicate with one’s colleagues.
However, two types of systems are sufficiently important that we can use them almost
exclusively: (1) liquid aqueous solutions and (2) ideal gas mixtures at atmospheric pressure.
In aqueous solutions we assume that the density is 1 g/cm3, the specific heat is 1 Cal/g K,
and at any solute concentration, pressure, or temperature there are -55 moles/liter of water.
In gases at one atmosphere and near room temperature we assume that the heat capacity per
mole is i R, the density is l/22.4 moles/liter, and all components obey the ideal gas equation
of state. Organic liquid solutions have constant properties within w-20%, and nonideal gas
solutions seldom have deviations larger than these.