Page 24 - The engineering of chemical reactions
P. 24
8 Introduction
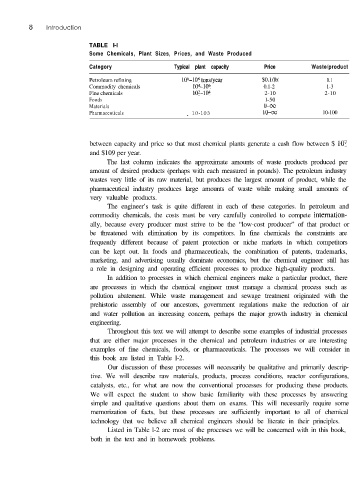
TABLE l-l
Some Chemicals, Plant Sizes, Prices, and Waste Produced
Category Typical plant capacity Price Waste/product
Petroleum refining 106-lo* tons/year $O,l/lb 0.1
Commodity chemicals 104-106 0.1-2 1-3
Fine chemicals lo*-104 2-10 2-10
Foods l-50
Materials O-00
Pharmaceuticals _ 10-103 lo-00 10-100
between capacity and price so that most chemical plants generate a cash flow between $ lo7
and $109 per year.
The last column indicates the approximate amounts of waste products produced per
amount of desired products (perhaps with each measured in pounds). The petroleum industry
wastes very little of its raw material, but produces the largest amount of product, while the
pharmaceutical industry produces large amounts of waste while making small amounts of
very valuable products.
The engineer’s task is quite different in each of these categories. In petroleum and
commodity chemicals, the costs must be very carefully controlled to compete intemation-
ally, because every producer must strive to be the “low-cost producer” of that product or
be threatened with elimination by its competitors. In fine chemicals the constraints are
frequently different because of patent protection or niche markets in which competitors
can be kept out. In foods and pharmaceuticals, the combination of patents, trademarks,
marketing, and advertising usually dominate economics, but the chemical engineer still has
a role in designing and operating efficient processes to produce high-quality products.
In addition to processes in which chemical engineers make a particular product, there
are processes in which the chemical engineer must manage a chemical process such as
pollution abatement. While waste management and sewage treatment originated with the
prehistoric assembly of our ancestors, government regulations make the reduction of air
and water pollution an increasing concern, perhaps the major growth industry in chemical
engineering.
Throughout this text we will attempt to describe some examples of industrial processes
that are either major processes in the chemical and petroleum industries or are interesting
examples of fine chemicals, foods, or pharmaceuticals. The processes we will consider in
this book are listed in Table l-2.
Our discussion of these processes will necessarily be qualitative and primarily descrip-
tive. We will describe raw materials, products, process conditions, reactor configurations,
catalysts, etc., for what are now the conventional processes for producing these products.
We will expect the student to show basic familiarity with these processes by answering
simple and qualitative questions about them on exams. This will necessarily require some
memorization of facts, but these processes are sufficiently important to all of chemical
technology that we believe all chemical engineers should be literate in their principles.
Listed in Table l-2 are most of the processes we will be concerned with in this book,
both in the text and in homework problems.