Page 70 - The engineering of chemical reactions
P. 70
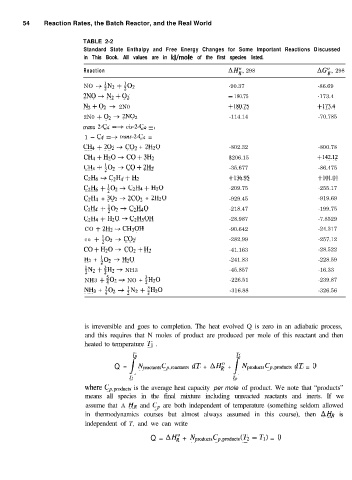
54 Reaction Rates, the Batch Reactor, and the Real World
TABLE 2-2
Standard State Enthalpy and Free Energy Changes for Some Important Reactions Discussed
in This Book. All values are in kJ/mole of the first species listed.
Reaction AH;, 298 AG;, 298
NO -+ ;N2 + $02 -90.37 -86.69
2NO+N2+02 - 180.75 -173.4
N2+02 -+ 2N0 +180.75 +173.4
2N0 + O2 + 2N02 -114.14 -70.785
tram 2-Q =+ cis-Z-C4 =,
1 - C4 =+ tram-2-C4 =
CH4 + 202 -+ CO2 + 2H20 -802.32 -800.78
Ch+H20+CO+3H2 $206.15 +142.1?
CH4+ ;02 + CO+2Hz -35.677 -86.475
C2H6 -+ C2H4 + Hz +136.95 +101.01
Cd&i + $2 + C2H4 + Hz0 -209.75 -255.17
C2H4 + 302 + 2C02 + 2H20 -929.45 -919.69
C2H4 + ;02 + C2H40 -218.47 -199.75
C2H4 + Hz0 + C2H5OH -28.987 -7.8529
CO + 2H2 -+ CH30H -90.642 -24.317
co + ;o, + CO2 -282.99 -257.12
CO+H20+ C02+H2 -41.163 -28.522
H2 + ;02 -+ Hz0 -241.83 -228.59
$N2 + 4H2 + NH3 -45.857 -16.33
NH3 + ;02 -+ NO + ;H20 -226.51 -239.87
NH3 + $02 + ;N2 + 5H20 -316.88 -326.56
is irreversible and goes to completion. The heat evolved Q is zero in an adiabatic process,
and this requires that N moles of product are produced per mole of this reactant and then
heated to temperature Ti .
TO T2
Q = NleactantSCp,Teactants dT + AH; + &mducts~p,products dT = 0
s s
Tl TO
where Cp,products is the average heat capacity per mole of product. We note that “products”
means all species in the final mixture including unreacted reactants and inerts. If we
assume that A HR and CP are both independent of temperature (something seldom allowed
in thermodynamics courses but almost always assumed in this course), then AHR is
independent of T, and we can write
Q = AH, + NproductsCp,products(T2 - Tl> = 0