Page 120 - Thermodynamics of Biochemical Reactions
P. 120
116 Chapter 6 Systems of Biochemical Reactions
Glc ATP ADP NADm NAQd p,
Glc 1 0 0 0 0 0
ATP 0 1 0 0 0 0
ADP 0 0 1 0 0 0
NADm 0 0 0 1 0 0
NADred 0 0 0 0 1 0
p, 0 0 0 0 0 1
G6P 1 1 -1 0 0 0
F6P 1 1 -I 0 0 0
F6BP 1 2 -2 0 0 0
DHAP 112 1 -1 0 0 0
13BPG 112 1 -1 1 -1 1
3PG 112 0 0 1 -1 1
2PG 112 0 0 I -1 1
PEP 112 0 0 1 -1 1
GAP 112 1 -1 0 0 0
PYr 1 J2 -1 1 1 -1 1
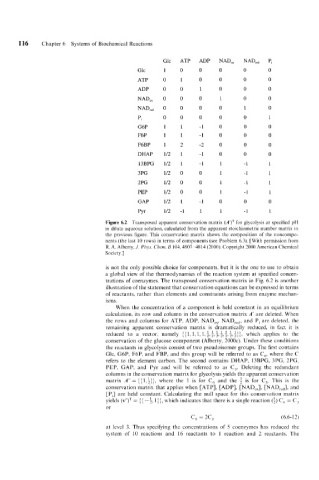
Figure 6.2 Transposed apparent conservation matrix (A’)’ for glycolysis at specified pH
in dilute aqueous solution, calculated from the apparent stoichiometric number matrix in
the previous figure. This conservation matrix shows the composition of the noncompo-
nents (the last 10 rows) in terms of components (see Problem 6.3). [With permission from
R. A. Alberty, J. Phys. Chern. B 104,4807-4814 (2000). Copyright 2000 American Chemical
Society.]
is not the only possible choice for components, but it is the one to use to obtain
a global view of the thermodynamics of the reaction system at specified concen-
trations of coenzymes. The transposed conservation matrix in Fig. 6.2 is another
illustration of the statement that conservation equations can be expressed in terms
of reactants, rather than elements and constraints arising from enzyme mechan-
isms.
When the concentration of a component is held constant in an equilibrium
calculation, its row and column in the conservation matrix A’ are deleted. When
the rows and columns for ATP, ADP, NAD,,, NAD,,,, and Pi are deleted, the
remaining apparent conservation matrix is dramatically reduced, in fact it is
11 11 1)
reduced to a vector, namely {{l, 1,1, l,i,~,z,z,~,~,~,}, which applies to the
1 1
conservation of the glucose component (Alberty, 2000~). Under these conditions
the reactants in glycolysis consist of two pseudoisomer groups. The first contains
Glc, G6P, F6P, and FBP, and this group will be referred to as C,, where the C
refers to the element carbon. The second contains DHAP, 13BPG, 3PG, 2PG.
PEP, GAP, and Pyr and will be referred to as C,. Deleting the redundant
columns in the conservation matrix for glycolysis yields the apparent conservation
matrix A” = {{l,;)), where the 1 is for C, and the is for C,. This is the
conservation matrix that applies when [ATP], [ADP], [NAD,,], [NAD,,,], and
[Pi] are held constant. Calculating the null space for this conservation matrix
yields (v”)~ = { { - ;, 1 j 1, which indicates that there is a single reaction (i) C, = C,
or
C, = 2c, (6.6- 12)
at level 3. Thus specifying the concentrations of 5 coenzymes has reduced the
system of 10 reactions and 16 reactants to 1 reaction and 2 reactants. The