Page 122 - Thermodynamics of Biochemical Reactions
P. 122
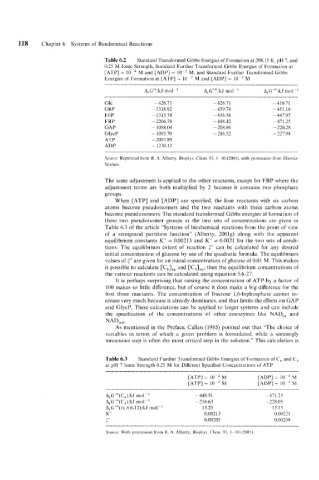
118 Chapter 6 Systems of Biochemical Reactions
Table 6.2 Standard Transformed Gibbs Energies of Formation at 298.15 K, pH 7, and
0.25 M Ionic Strength, Standard Further Transformed Gibbs Energies of Formation at
[ATP] = M and [ADP] = lo-* M. and Standard Further Transformed Gibbs
Energies of Formation at [ATP] = lo-* M and [ADP] = lo-* M
A,G’”/kJ mo1-l A,G”’/kJ rno1-I A,G’JO/kJ mo1-l
Glc - 426.7 1 - 426.7 1 - 41 6.7 1
G6P - 1318.92 -439.74 -451.16
F6P - 1315.74 - 436.56 - 447.97
FBP - 2206.78 - 448.42 -471.25
GAP - 1088.04 - 208.86 - 220.28
GlycP - 1095.70 -216.52 - 227.94
ATP - 2097.89
ADP - 1230.12
Source: Reprinted from R. A. Alberty, Biophjs. Clzem. 93, 1-10 (2001), with permission from Elsevicr
Science.
The same adjustment is applied to the other reactants, except for FBP where the
adjustment terms are both multiplied by 2 because it contains two phosphate
groups.
When [ATP] and [ADP] are specified, the four reactants with six carbon
atoms become pseudoisomers and the two reactants with three carbon atoms
become pseudoisomers. The standard transformed Gibbs energies of formation of
these two pseudoisomer groups at the two sets of concentrations are given in
Table 6.3 of the article “Systems of biochemical reactions from the point of view
of a semigrand partition function” (Alberty, 2001g) along with the apparent
equilibrium constants K” = 0.00213 and K“ = 0.0021 for the two sets of condi-
tions. The equilibrium extent of reaction (I‘ can be calculated for any desired
initial concentration of glucose by use of the quadratic formula. The equilibrium
values of (3’ are given for an initial concentration of glucose of 0.01 M. This makes
it possible to calculate [CJeq and [C,],,; then the equilibrium concentrations of
the various reactants can be calculated using equation 5.6-27.
It is perhaps surprising that raising the concentration of ATP by a factor of
100 makes so little difference, but of course it does make a big difference for the
first three reactants. The concentration of fructose 1,6-biphosphate cannot in-
crease very much because it already dominates, and that limits the effects on GAP
and GlycP. These calculations can be applied to larger systems and can include
the specification of the concentrations of other coenzymes like NAD,, and
NADre,.
As mentioned in the Preface, Callen (1985) pointed out that “The choice of
variables in terms of which a given problem is formulated, while a seemingly
innocuous step is often the most critical step in the solution.” This calculation is
Table 6.3 Standard Further Transformed Gibbs Energies of Formation of C, and C,
at pH 7 Ionic Strength 0.25 M for Different Specified Concentrations of ATP
[ATP] = M [ADP] = M
[ATP] = M [ADP] = M
Af G ““(C,)/kJ mol- -448.51 - 471.25
AtG’“(C,)/kJ mol- ’ -216.63 - 228.05
Ar G”’(rx 6.6- 12)/kJ inol- 15.25 15.15
K “ 0.00213 0.0022 1
;,,
i 0.00205 0.00209
Source: With permission from R. A. Alberty, Biophys. Chem. 93. 1- 10 (2001).