Page 127 - Thermodynamics of Biochemical Reactions
P. 127
124 Chapter 7 Thermodynamics of the Binding of Ligands by Proteins
t -- -
-- _- - --.
LO21
5x10-" 0.00001 0.000015 0.00002
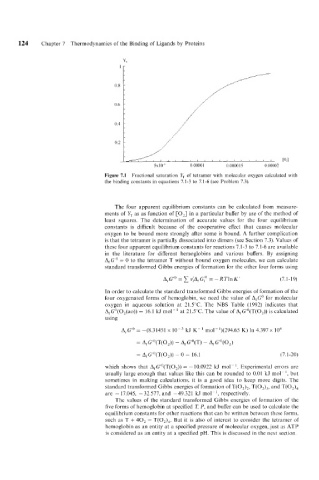
Figure 7.1 Fractional saturation Y, of tetramer with molecular oxygen calculated with
the binding constants in equations 7.1-3 to 7.1-6 (see Problem 7.3).
The four apparent equilibrium constants can be calculated from measure-
ments of YT as as function of [O,] in a particular buffer by use of the method of
least squares. The determination of accurate values for the four equilibrium
constants is difficult because of the cooperative effect that causes molecular
oxygen to be bound more strongly after some is bound. A further complication
is that the tetramer is partially dissociated into dimers (see Section 7.3). Values of
these four apparent equilibrium constants for reactions 7.1-3 to 7.1-6 are available
in the literature for different hemoglobins and various buffers. By assigning
A,G" = 0 to the tetramer T without bound oxygen molecules, we can calculate
standard transformed Gibbs energies of formation for the other four forms using
A,G" = V~A~G;" = - RTln K' (7.1 - 19)
In order to calculate the standard transformed Gibbs energies of formation of the
four oxygenated forms of hemoglobin, we need the value of A,G" for molecular
oxygen in aqueous solution at 21.5'C. The NBS Table (1992) indicates that
ArG"(O,(ao)) = 16.1 kJ mol-' at 21.5"C. The value of.A,G'"(T(O,)) is calculated
using
A,G" = -(8.31451 x kJ K-l mol-')(294.65 K) In 4.397 x 10"
= A,G'"(T(O,)) - AfG"(T) ~ AfG'O(O,)
= A,G"(T(O,)) ~ 0 - 16.1 (7.1-20)
which shows that AfG'"(T(O,)) = - 10.0922 kJ mol ~ '. Experimental errors are
usually large enough that values like this can be rounded to 0.01 kJ mol-', but
sometimes in making calculations. it is a good idea to keep more digits. The
standard transformed Gibbs energies of formation of T(O,),, T(02)3. and T(O,),
are - 17.045, -32.577, and -49.321 kJ mol-', respectively.
The values of the standard transformed Gibbs energies of formation of the
five forms of hemoglobin at specified 7: P. and buffer can be used to calculate the
equilibrium constants for other reactions that can be written between these forms.
such as T + 40, = T(O,),. But it is also of interest to consider the tetramer of
hemoglobin as an entity at a specified pressure of molecular oxygen, just as ATP
is considered as an entity at a specified pH. This is discussed in the next section.