Page 160 - Thermodynamics of Biochemical Reactions
P. 160
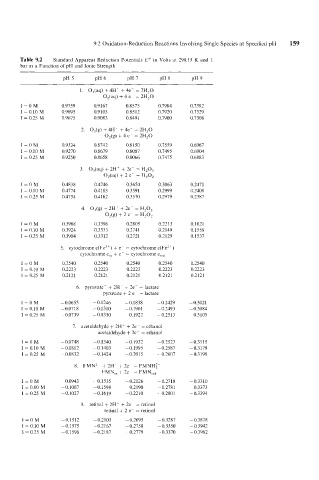
9.2 Oxidation-Reduction Reactions Involving Single Species at Specified pH 159
Table 9.2 Standard Apparent Reduction Potentials E'" in Volts at 298.15 K and 1
bar as a Function of pH and Ionic Strength
1. O,(aq) + 4Ht + 4e- = 2H,O
OJaq) + 4 e- = 2H,O
I=OM 0.9759 0.9167 0.8575 0.7984 0.7392
I = 0.10 M 0.9695 0.9 103 0.8512 0.7920 0.7329
I = 0.25 M 0.9675 0.9083 0.849 1 0.7900 0.7308
2. O,(g) + 4H' + 4e- = 2H,O
O,(g) + 4 e- = 2H,O
I=OM 0.9334 0.8742 0.8 150 0.7559 0.6967
I = 0.10 M 0.9270 0.8679 0.8087 0.7495 0.6904
I = 0.25 M 0.9250 0.8658 0.8066 0.7475 0.6883
3. O,(aq) + 2H+ + 2e- = H,O,
O,(aq) + 2 e- = H,O,
I=OM 0.4838 0.4246 0.3654 0.3063 0.2471
I = 0.10 M 0.4774 0.4183 0.3591 0.2999 0.2408
I = 0.25 M 0.4754 0.4162 0.3570 0.2979 0.2387
4. O,(g) + 2H+ + 2e = H,O,
O,(g) + 2 e = H,O,
I=OM 0.3988 0.3396 0.2805 0.2213 0.1621
I = 0.10 M 0.3924 0.3333 0.2741 0.2 149 0.1558
I = 0.25 M 0.3904 0.3312 0.2721 0.2129 0.1537
5. cytochrome c(Fe3') + e- = cytochrome c(Fe2')
cytochrome c,, + c ~ = cytochrome cred
I=OM 0.2540 0.2540 0.2540 0.2540 0.2540
I = 0.10 M 0.2223 0.2223 0.2223 0.2223 0.2223
I = 0.25 M 0.2121 0.2121 0.2121 0.2121 0.2121
6. pyruvate- + 2H' + 2e- = lactate
pyruvate + 2 e- = lactate
T=OM -0.0655 - 0.1246 - 0.1838 - 0.2429 - 0.3021
I = 0.10 M -0.0718 -0.1310 - 0.1901 -0.2493 - 0.3084
I = 0.25 M -0.0739 -0.1330 -0.1922 - 0.251 3 -0.3105
7. acetaldehyde + 2H' + 2e- = ethanol
acetaldehyde + 2e- = ethanol
I=OM - 0.0748 - 0.1340 -0.1932 -0.2523 -0.3115
I = 0.10 M -0.081 2 -0.1403 -0.1995 -0.2587 - 0.3 179
I = 0.25 M -0.0832 - 0.1424 -0.2015 -0.2607 -0.3199
8. FMN2- + 2H' + 2e- = FMNH:-
FMN,, + 2e- = FMN,,,
I=OM - 0.0943 -0.1535 -0.2126 -0.2718 -0.3310
I = 0.10 M -0.1007 -0.1 59 8 -0.2190 -0.2781 -0.3373
I = 0.25 M -0.1027 - 0.1619 -0.2210 -0.2801 -0.3394
9. retinal + 2H' + 2e- = retinol
retinal + 2 e- = retinol
I=OM -0.1512 -0.2103 - 0.2695 -0.3287 -0.3878
I = 0.10 M -0.1575 -0.2167 -0.2758 -0.3350 - 0.3942
I = 0.25 M -0.1596 -0.2187 - 0.2779 -0.3370 - 0.3962