Page 162 - Thermodynamics of Biochemical Reactions
P. 162
9.2 Oxidation-Reduction Reactions Involving Single Species at Specified pH 161
E"'l v
lr
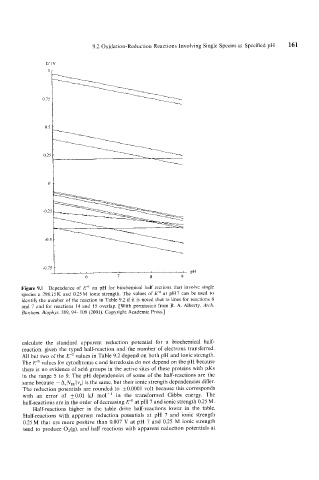
Figure 9.1 Dependence of E'' on pH for biochemical half rections that involve single
species a 298.15K and 0.25M ionic strength. The values of E'' at pH7 can be used to
identify the number of the reaction in Table 9.2 if it is noted that te lines for reactions 6
and 7 and for reactions 14 and 15 overlap. [With permission from R. A. Alberty, Arch.
Bioclzem. Biophys. 389, 94- 109 (2001). Copyright Academic Press.]
calculate the standard apparent reduction potential for a biochemical half-
reaction, given the typed half-reaction and the number of electrons transferred.
All but two of the E'O values in Table 9.2 depend on both pH and ionic strength.
The E'O values for cytochrome c and ferredoxin do not depend on the pH because
there is no evidence of acid groups in the active sites of these proteins with pKs
in the range 5 to 9. The pH dependencies of some of the half-reactions are the
same because -ArNH/Iv,I is the same, but their ionic strength dependencies differ.
The reduction potentials are rounded to 0.0001 volt because this corresponds
with an error of kO.01 kJ mol-' in the transformed Gibbs energy. The
half-reactions are in the order of decreasing E'O at pH 7 and ionic strength 0.25 M.
Half-reactions higher in the table drive half-reactions lower in the table.
Half-reactions with apparent reduction potentials at pH 7 and ionic strength
0.25M that are more positive than 0.807 V at pH 7 and 0.25 M ionic strength
tend to produce O,(g), and half reactions with apparent reduction potentials at