Page 165 - Thermodynamics of Biochemical Reactions
P. 165
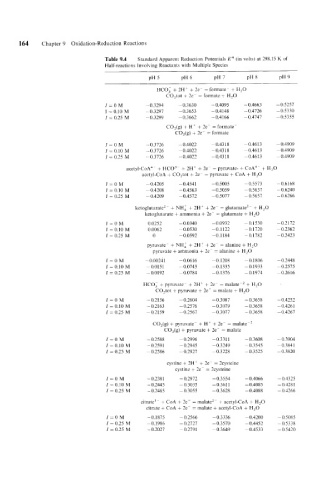
164 Chapter 9 Oxidation-Reduction Reactions
Table 9.4 Standard Apparent Reduction Potentials E'" (in volts) at 298.15 K of
Half-reactions Involving Reactants with Multiple Species
HC0.i + 2H' + 2e- = formate- + H,O
C0,tot + 2e- = formate + H,O
I=OM -0.3294 -0.3630 - 0.4095 - 0.4663 -0.5257
1 = 0.10 M -0.3297 -0.3653 -0.4148 - 0.4726 -0.5330
I = 0.25 M ~~ 0.3299 -0.3662 -0.4166 - 0.4747 -0.5355
CO,(g) + H' + 2e- = formate
CO,(g) + 2e- = formate
/=OM -0.3726 - 0.4022 -0.4318 -0.4613 - 0.4909
I = 0.10 M -0.3726 - 0.4022 - 0.43 18 - 0.461 3 -0.4909
I = 0.25 M -0.3726 - 0.4022 - 0.43 18 -0.461 3 - 0.4909
acetyl-CoA4- + HC03- + 2H' + 2e- = pyruvate- + CoA"- + H,O
acctyl-CoA + C0,tot + 2e- = pyruvatc + CoA + H,O
I=OM - 0.4205 -0.4541 - 0.5005 -0.5573 -0.6168
I = 0.10 M - 0.4208 -0.4563 - 0.5059 - 0.5637 -0.6240
I = 0.25 M - 0.4209 -0.4572 - 0.5077 - 0.5657 - 0.6266
ketog1utarate'- + NH,' + 2H' + 2e- = glutamate'- + H,O
ketoglutarate + ammonia + 2e- = glutamate + H,O
I=OM 0.0252 - 0.0340 - 0.0932 - 0.1530 -0.2112
I = 0.10 M 0.0062 - 0.0530 -0.1122 - 0.1720 - 0.2362
I = 0.25 M 0 - 0.0592 - 0.1 184 -0.1782 - 0.2423
pyruvate- + NH: + 2H' + 2e- = alanine + H,O
pyruvate + ammonia + 2e- = alanine + H,O
I=OM -0.00241 - 0.06 16 -0.1208 -0.1806 - 0.2448
I = 0.10 M -0.0151 - 0.0743 -0.1335 -0.1933 -0.2515
I = 0.25 M -0,0192 -0.0784 -0.1376 -0.1974 - 0.2616
HCO, + pyruvate- + 2H+ + 2e- = malate-, + H,O
C0,tot + pyruvate + 2c- = malate + H,O
/=OM -~-0.2156 - 0.2604 -0.3087 -0.3658 -0.4252
I = 0.10 M -0.2163 - 0.2576 - 0.3079 -0.3658 - 0.426 1
I = 0.25 M -0.2159 -0.2567 -0.3071 - 0.3658 - 0.4267
CO,(g) + pyruvate- + H' + 2e- = malate-,
CO,(g) + pyruvate + 2e- = malate
I=OM -0.2588 - 0.2996 -0.3311 -0.3608 - 0.3904
I = 0.10 M -0.2591 -0.2945 - 0.3249 -0.3545 - 0.384 1
I = 0.25 M -0.2586 -0.2927 -0.3228 -0.3525 -0.3820
cystine + 2H' + 2e- = 2cysteine
cystine + 2c- = 2cysteine
I=OM -0.2381 -0.2972 -0.3554 - 0.4066 - 0.4325
I = 0.10 M - 0.2445 -0.3035 -0.361 1 - 0.4085 - 0.428 1
I = 0.25 M - 0.2465 -0.3055 -0.3628 - 0.4088 -0.4266
citrate" + CoA + 2e- = malate,- + acetyl-CoA + H,O
citrate + CoA + 2e- = malate + acctyl-CoA + H,O
I=OM - 0.1875 - 0.2566 -0.3336 - 0.4200 - 0.5085
I = 0.25 M - 0.1986 -0.2127 -0.3570 - 0.4452 -0.5338
I = 0.25 M - 0.2021 - 0.279 1 - 0.3649 - 0.4533 - 0.5420