Page 169 - Thermodynamics of Biochemical Reactions
P. 169
168 Chapter 9 Oxidation-Reduction Reactions
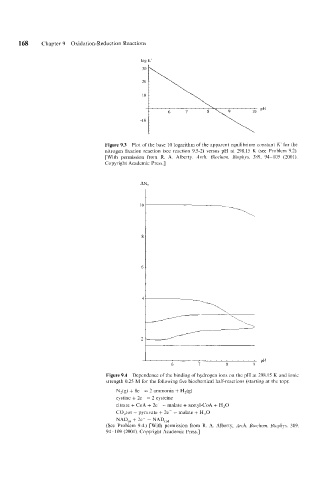
log K'
Figure 9.3 Plot of the base 10 logarithm of the apparent equilibrium constant K' for the
nitrogen fixation reaction (see reaction 9.5-2) versus pH at 298.15 K (see Problem 9.2).
[With permissioti from R. A. Alberty, Arch. Biochem. Biophys. 389, 94- 109 (2001).
Copyright Academic Press.]
-- --- --.
--
--.
--- ---
_-
_- --.
--.
--
PH
6 7 8 9 I
Figure 9.4 Dependence of the binding of hydrogen ions on the pH at 298.15 K and ionic
strength 0.25 M for the following five biochemical half-reactions (starting at the top):
N,(g) + 8e- =: 2 ammonia + H2(g)
cystine + 2e- = 2 cysteine
citrate + CoA + 2e- = malate + acetyl-CoA + H,O
C0,tot + pyruvate + 2e- = malate + H,O
NAD,, + 2e- = NAD,,,
(See Problem 9.4.) [With pcrmission from R. A. Alberty, Arch. Biochem. Biophj,s. 389.
94 109 (2001). Copyright Academic Press.]