Page 170 - Thermodynamics of Biochemical Reactions
P. 170
9.6 Changes in the Binding of Hydrogen Ions in Half Reactions at Specified pH 169
I ......... I .... I ....
6 7 8
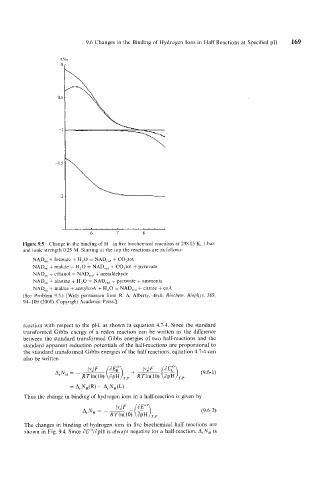
Figure 9.5 Change in the binding of H+ in five biochemical reactions at 298.15 K, 1 bar,
and ionic strength 0.25 M. Starting at the top the reactions are as follows:
NAD,, + formate + H,O = NAD,,, + C0,tot
NAD,, + malate + H,O = NAD,,, + C0,tot + pyruvate
NAD,, + ethanol = NAD,,, + acetaldehyde
NAD,, + alanine + H,O = NAD,,, + pyruvate + ammonia
NAD,, + malate + acetylcoA + H,O = NAD,,, + citrate + coA
(See Problem 9.5.) [With permission from R. A. Alberty, Arch. Biochern. Biophps. 389,
94- 109 (2001). Copyright Academic Press.]
reaction with respect to the pH, as shown in equation 4.7-4. Since the standard
transformed Gibbs energy of a redox reaction can be written as the difference
between the standard transformed Gibbs energies of two half-reactions and the
standard apparent reduction potentials of the half-reactions are proportional to
the standard transformed Gibbs energies of the half reactions, equation 4.7-4 can
also be written
(g) (9.6-1)
RTln(l0) 8pH T,P
= ArNH(R) - ArNH(L)
Thus the change in binding of hydrogen ions in a half-reaction is given by
ArNH = - (9.6-2)
The changes in binding of hydrogen ions in five biochemical half reactions are
shown in Fig. 9.4. Since i?E''/i;pH is always negative for a half-reaction, ArNH is