Page 167 - Thermodynamics of Biochemical Reactions
P. 167
166 Chapter 9 Oxidation-Reduction Reactions
t-' IV
0
1
-0
2
-0
3
-0
-0 4
5
-0
-0 6
-...
' PH
6 I 8 9
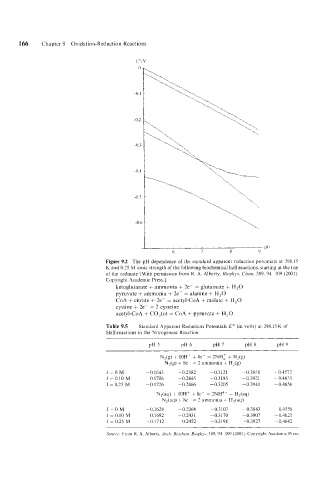
Figure 9.2 The pH dependeiice of the standard apparent reduction potentials at 298.15
K and 0.25 M ionic strength of the following biochemical half reactions, starting at the top
of the ordinate [With permission from R. A. Alberty, Biophys. Clzrrn. 389, 94-109 (2001).
Copyright Academic Press.]:
ketoglutarate + ammonia + 2e- = glutamate + H,O
pyruvate + ammonia + 2e- = alanine + H,O
CoA + citrate + 2e- = acetyl-CoA + malate + H,O
cystine + 2e~- = 2 cysteine
acetyl-CoA + C0,tot = CoA + pyruvate + H,O
Table 9.5 Standard Apparent Reduction Potentials E'" (in volts) at 298.15 K of
Half-reactions in the Nitrogcnase Reaction
N2(g) + IOH ' + 8e- = 2NHd + H,(g)
N,(g) + 8e- = 2 ammonia + H,(g)
I=OM -- 0.1643 - 0.2382 - 0.3 121 --0.3858 -0.4512
I = 0.10 M -0,1706 - 0.2445 - 0.3 185 -0.3921 - 0.4635
I = 0.25 M --0,1726 - 0.2466 -0.3205 - 0.394 1 - 0.4656
N,(aq) + 10H' + 8e- = 2NH"+ + H,(aq)
N,(aq) + 8e- = 2 ammonia + H,(aq)
I=OM -0.1628 - 0.2368 - 0.3 I 07 -0.3843 -0.4558
I = 0.10 M ~- 0.1692 - 0.243 1 -0.3 170 - 0.3907 - 0.462 1
I = 0.25 M -- 0.17 12 -0.2452 -0.3191 -0.3921 -- 0.4642
Soirrce: From R. A. Alherty, Arch. Bioc~hrr~. Biophj,\. 389, 94 109 (2001). Copyright Academic Press.