Page 286 - Bird R.B. Transport phenomena
P. 286
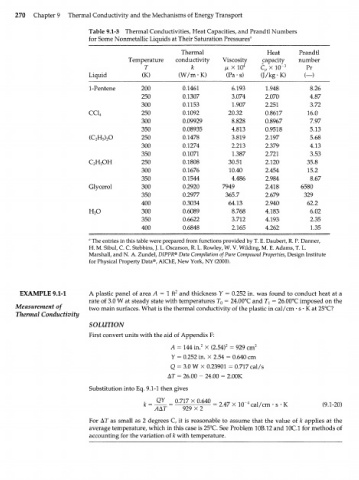
270 Chapter 9 Thermal Conductivity and the Mechanisms of Energy Transport
Table 9.1-3 Thermal Conductivities, Heat Capacities, and Prandtl Numbers
for Some Nonmetallic Liquids at Their Saturation Pressures"
Thermal Heat Prandtl
Temperature conductivity Viscosity capacity number
T к /xXlO 4 C p X 10" 3 Pr
Liquid (K) (W/m • K) (Pa • s) (J/kg-K) (—)
1-Pentene 200 0.1461 6.193 1.948 8.26
250 0.1307 3.074 2.070 4.87
300 0.1153 1.907 2.251 3.72
CC1 4 250 0.1092 20.32 0.8617 16.0
300 0.09929 8.828 0.8967 7.97
350 0.08935 4.813 0.9518 5.13
(C H ) O 250 0.1478 3.819 2.197 5.68
5 2
2
300 0.1274 2.213 2.379 4.13
350 0.1071 1.387 2.721 3.53
C H OH 250 0.1808 30.51 2.120 35.8
2 5
300 0.1676 10.40 2.454 15.2
350 0.1544 4.486 2.984 8.67
Glycerol 300 0.2920 7949 2.418 6580
350 0.2977 365.7 2.679 329
400 0.3034 64.13 2.940 62.2
H O 300 0.6089 8.768 4.183 6.02
2
350 0.6622 3.712 4.193 2.35
400 0.6848 2.165 4.262 1.35
a
The entries in this table were prepared from functions provided by Т. Е. Daubert, R.P. Danner,
H. M. Sibul, Г Г Stebbins,J.L. Oscarson, R. L. Rowley, W. V. Wildi]ng, M. E. Adams, T. L.
Marshall, and N. A. Zundel, DIPPR® Data Compilation of Pure Compound Properties, Design Institute
for Physical Property Data®, AIChE, New York, NY (2000).
EXAMPLE 9.1-1 A plastic panel of area A = 1 ft 2 and thickness У = 0.252 in. was found to conduct heat at a
rate of 3.0 W at steady state with temperatures T o = 24.00°C and T } = 26.00°C imposed on the
Measurement of two main surfaces. What is the thermal conductivity of the plastic in cal/cm • s • К at 25°C?
Thermal Conductivity
SOLUTION
First convert units with the aid of Appendix F:
A = 144 in. 2 X (2.54) 2 = 929 cm 2
У = 0.252 in. X 2.54 = 0.640 cm
Q = 3.0 W X 0.23901 = 0.717 cal/s
AT = 26.00 - 24.00 = 2.00K
Substitution into Eq. 9.1-1 then gives
0.717 X 0.640 - i/
k = = 929X2 = О Л 7 ч / 1 П 4 cal/cm • s K (9.1-20)
For AT as small as 2 degrees C, it is reasonable to assume that the value of к applies at the
average temperature, which in this case is 25°C. See Problem 10B.12 and 10C.1 for methods of
accounting for the variation of к with temperature.