Page 293 - Bird R.B. Transport phenomena
P. 293
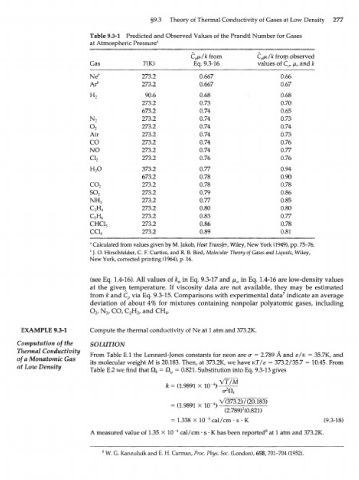
§9.3 Theory of Thermal Conductivity of Gases at Low Density 277
Table 9.3-1 Predicted and Observed Values of the Prandtl Number for Gases
at Atmospheric Pressure"
С /л/к from С /л/к from observed
р
р
Gas TOQ Eq. 9.3-16 values of C , /x, and к
p
Ne b 273.2 0.667 0.66
Ar b 273.2 0.667 0.67
H 2 90.6 0.68 0.68
273.2 0.73 0.70
673.2 0.74 0.65
N 2 273.2 0.74 0.73
o 273.2 0.74 0.74
2
Air 273.2 0.74 0.73
CO 273.2 0.74 0.76
NO 273.2 0.74 0.77
ci 273.2 0.76 0.76
2
H O 373.2 0.77 0.94
2
673.2 0.78 0.90
co 273.2 0.78 0.78
so 2 273.2 0.79 0.86
NH 2 3 273.2 0.77 0.85
с н 273.2 0.80 0.80
2
C H 4 273.2 0.83 0.77
2 6
CHC1 3 273.2 0.86 0.78
CC1 273.2 0.89 0.81
4
" Calculated from values given by M. Jakob, Heat Transfer, Wiley, New York (1949), pp. 75-76.
b
]. O. Hirschfelder, C. F. Curtiss, and R. B. Bird, Molecular Theory of Gases and Liquids, Wiley,
New York, corrected printing (1964), p. 16.
(see Eq. 1.4-16). All values of k a in Eq. 9.3-17 and fi a in Eq. 1.4-16 are low-density values
at the given temperature. If viscosity data are not available, they may be estimated
7
from к and C p via Eq. 9.3-15. Comparisons with experimental data indicate an average
deviation of about 4% for mixtures containing nonpolar polyatomic gases, including
O , N CO, C H and CH .
2 /
4
2
2
2 /
EXAMPLE 9.3-1 Compute the thermal conductivity of Ne at 1 atm and 373.2K.
Computation of the SOLUTION
Thermal Conductivity From Table E.I the Lennard-Jones constants for neon are cr = 2.789 A and е/к = 35.7K, and
of a Monatomic Gas its molecular weight M is 20.183. Then, at 373.2K, we have кТ/е = 373.2/35.7 = 10.45. From
at Low Density
Table E.2 we find that u k = % = 0.821. Substitution into Eq. 9.3-13 gives
It =(1.9891 X10" )-
4
= (1.9891 X 0-^) V ( 3 7 3 - 2 ) / Q 0 - 1 8 3 )
2 2
(2.789) (0.821)
4
= 1.338 X 10~ cal/cm • s • К (9.3-18)
8
A measured value of 1.35 X 10 ~ cal/cm • s • К has been reported at 1 atm and 373.2K.
4
W. G. Kannuluik and E. H. Carman, Proc. Phys. Soc. (London), 65B, 701-704 (1952).
s