Page 537 - Bird R.B. Transport phenomena
P. 537
§17.1 Fick's Law of Binary Diffusion (Molecular Mass Transport) 517
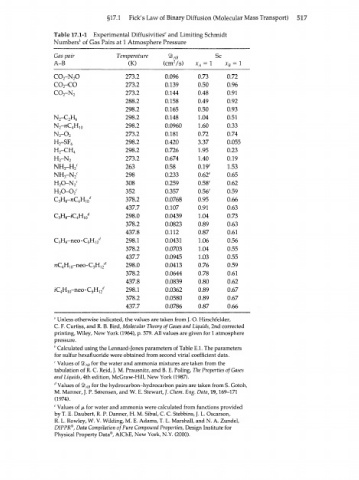
Table 17.1-1 Experimental Diffusivities" and Limiting Schmidt
Numbers'' of Gas Pairs at 1 Atmosphere Pressure
Gas pair Temperature ®AB Sc
A-B (K) (cm7s) x A = 1 х в = 1
CO -N O 273.2 0.096 0.73 0.72
2 2
CO -CO 273.2 0.139 0.50 0.96
2
CO -N 273.2 0.144 0.48 0.91
2 2
288.2 0.158 0.49 0.92
298.2 0.165 0.50 0.93
N -C H 6 298.2 0.148 1.04 0.51
2
2
N -nC H 1 0 298.2 0.0960 1.60 0.33
2
4
N -O 273.2 0.181 0.72 0.74
2 2
H -SF 6 298.2 0.420 3.37 0.055
2
H -CH 4 298.2 0.726 1.95 0.23
2
H -N 273.2 0.674 1.40 0.19
2 2
NH -H C 263 0.58 0.19 е 1.53
3 2
NH -N C 298 0.233 0.62 е 0.65
3 2
H O-N C 308 0.259 0.58 е 0.62
2 2
H O-O C 352 0.357 0.56 е 0.59
2 2
C H -nC H ' y 378.2 0.0768 0.95 0.66
3
8
4
10
437.7 0.107 0.91 0.63
C H -z'C H 10 d 298.0 0.0439 1.04 0.73
8
4
3
378.2 0.0823 0.89 0.63
437.8 0.112 0.87 0.61
C H -neo-C H rf 298.1 0.0431 1.06 0.56
3 8 5 12
378.2 0.0703 1.04 0.55
437.7 0.0945 1.03 0.55
wC H -neo-C H rf 298.0 0.0413 0.76 0.59
4 10 5 12
378.2 0.0644 0.78 0.61
437.8 0.0839 0.80 0.62
/C H -neo-C H rf 298.1 0.0362 0.89 0.67
4 10 5 12
378.2 0.0580 0.89 0.67
437.7 0.0786 0.87 0.66
" Unless otherwise indicated, the values are taken from J. O. Hirschfelder,
C. F. Curtiss, and R. B. Bird, Molecular Theory of Gases and Liquids, 2nd corrected
printing, Wiley, New York (1964), p. 579. All values are given for 1 atmosphere
pressure.
/7 Calculated using the Lennard-Jones parameters of Table E.I. The parameters
for sulfur hexafluoride were obtained from second virial coefficient data.
c Values of %b for the water and ammonia mixtures are taken from the
AB
tabulation of R. C. Reid, J. M. Prausnitz, and В. Е. Poling, The Properties of Gases
and Liquids, 4th edition, McGraw-Hill, New York (1987).
d
Values of ЯЬ for the hydrocarbon-hydrocarbon pairs are taken from S. Gotoh,
АВ
M. Manner, J. P. Stfrensen, and W. E. Stewart, /. Chem. Eng. Data, 19,169-171
(1974).
e
Values of /x for water and ammonia were calculated from functions provided
by T. E. Daubert, R. P. Danner, H. M. Sibul, С. С Stebbins, J. L. Oscarson,
R. L. Rowley, W. V. Wilding, M. E. Adams, T. L. Marshall, and N. A. Zundel,
DIPPR®, Data Compilation of Pure Compound Properties, Design Institute for
Physical Property Data®, AIChE, New York, N.Y. (2000).