Page 538 - Bird R.B. Transport phenomena
P. 538
518 Chapter 17 Diffusivity and the Mechanisms of Mass Transport
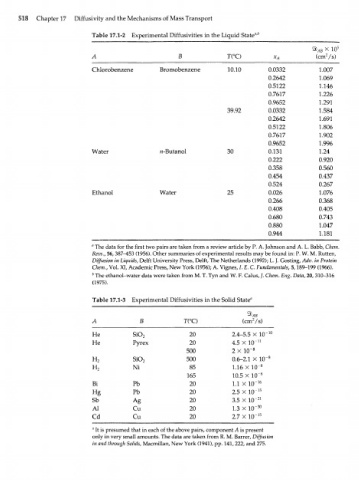
Table 17.1-2 Experimental Diffusivities in the Liquid Stated
ЯЬ АВ X Ю 5
2
T(°C) (cm /s)
Chlorobenzene Bromobenzene 10.10 0.0332 1.007
0.2642 1.069
0.5122 1.146
0.7617 1.226
0.9652 1.291
39.92 0.0332 1.584
0.2642 1.691
0.5122 1.806
0.7617 1.902
0.9652 1.996
Water н-Butanol 30 0.131 1.24
0.222 0.920
0.358 0.560
0.454 0.437
0.524 0.267
Ethanol Water 25 0.026 1.076
0.266 0.368
0.408 0.405
0.680 0.743
0.880 1.047
0.944 1.181
" The data for the first two pairs are taken from a review article by P. A. Johnson and A. L. Babb, Chem.
Revs., 56, 387-^153 (1956). Other summaries of experimental results may be found in: P. W. M. Rutten,
Diffusion in Liquids, Delft University Press, Delft, The Netherlands (1992); L. J. Gosting, Adv. in Protein
Chem., Vol. XI, Academic Press, New York (1956); A. Vignes, /. E. С Fundamentals, 5,189-199 (1966).
b The ethanol-water data were taken from M. T. Tyn and W. F. Calus, /. Chem. Eng. Data, 20, 310-316
(1975).
Table 17.1-3 Experimental Diffusivities in the Solid State"
А В T(°C) (cmVs)
Не SiO 20 2.4-5.5 X 10~ 10
2
Не Pyrex 20 4.5 X 10~ n
500 2 X 10~ 8
н 2 SiO 2 500 0.6-2.1 X 10~ 8
н Ni 85 1.16 X10" 8
2
165 10.5 X 10" 8
Bi Pb 20 1.1 x 10~ 16
Hg Pb 20 2.5 X 10~ 15
Sb Ag 20 3.5 X 10" 21
Al Cu 20 1.3 X 10~ 30
Cd Cu 20 2.7 X 10~ 15
" It is presumed that in each of the above pairs, component A is present
only in very small amounts. The data are taken from R. M. Barrer, Diffusion
in and through Solids, Macmillan, New York (1941), pp. 141, 222, and 275.