Page 638 - Bird R.B. Transport phenomena
P. 638
618 Chapter 20 Concentration Distributions with More Than One Independent Variable
The absence of a characteristic length in this problem, and the fact that c B = c 6 x both at
t = 0 and z = oo, suggests trying a combination of variables. Comparison with the previous
example (without the v* term) suggests the following trial solutions:
for 0 < z < z (t) (20.1-33)
R
for z (t) < z < oo (20.1-34)
R
These functions satisfy the differential equations, and if the constants of integration, C ] to C ,
4
can be so chosen that the initial and boundary conditions are satisfied, we will have the com-
plete solution to the problem.
Application of the initial condition and the first three boundary conditions permits the
evaluation of the integration constants in terms of z (t), thereby giving
R
erffc/
for 0 < z < z (t) (20.1-35)
R
for z (t) < z < oo (20.1-36)
R
B.C. 5 is then automatically satisfied. Finally, insertion of these solutions into B.C. 4 gives the
following implicit equation from which z (t) can be obtained:
R
(20.1-37)
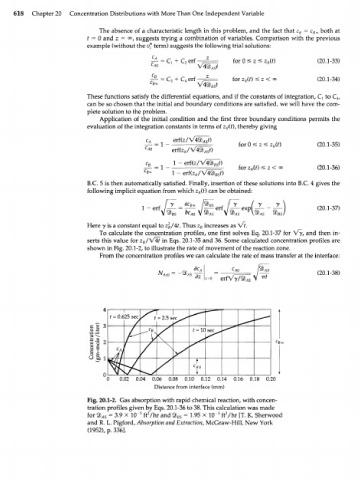
Here у is a constant equal to z\/\t. Thus z increases as Vf.
R
To calculate the concentration profiles, one first solves Eq. 20.1-37 for Vy, and then in-
/
serts this value for z /\ 4t in Eqs. 20.1-35 and 36. Some calculated concentration profiles are
R
shown in Fig. 20.1-2, to illustrate the rate of movement of the reaction zone.
From the concentration profiles we can calculate the rate of mass transfer at the interface:
dc A
(20.1-38)
si
t = o.t 25 sec = 2.5s .
^ ^
f = 10s e c ^ * ^
is a» /
ft<
f V 1
ok 41
t
c
A0
0
0 0.02 0.04 0.06 0.08 0.10 0.12 0.14 0.16 0.18 0.20
Distance from interface (mm)
Fig. 20.1-2. Gas absorption with rapid chemical reaction, with concen-
tration profiles given by Eqs. 20.1-36 to 38. This calculation was made
2
5
2
5
for 4b = 3.9 X 10" ft /hr and 9) = 1.95 X 10" ft /hr [Т. К. Sherwood
AS BS
and R. L. Pigford, Absorption and Extraction, McGraw-Hill, New York
(1952), p. 336].